EuroQol 5-Dimensional (EQ-5D) Instrument
(Redirected from EuroQol 5-Dimensional (EQ-5D) Questionnaire)
Jump to navigation
Jump to search
A EuroQol 5-Dimensional (EQ-5D) Instrument is a quality-of-life self-reported COA.
- Context:
- It can range from being an English EQ-5D Questionnaire to being a Chinese EQ-5D Questionnaire to being ...
- …
- Example(s):
- Counter-Example(s):
- See: Questionnaire.
References
2021
- https://euroqol.org/publications/user-guides/
- QUOTE: The EQ-5D User Guides have been developed to provide users basic information about how to use the EQ-5D instruments. The EQ-5D User Guide is available for the EQ-5D-3L, the EQ-5D-5L and the EQ-5D-Y versions:
2021
- (Wikipedia, 2021) ⇒ https://en.wikipedia.org/wiki/EQ-5D Retrieved:2021-11-30.
- EQ-5D is a standardised measure of health-related quality of life developed by the EuroQol Group to provide a simple, generic questionnaire for use in clinical and economic appraisal and population health surveys. EQ-5D assesses health status in terms of five dimensions of health and is considered a ‘generic’ questionnaire because these dimensions are not specific to any one patient group or health condition. EQ-5D can also be referred to as a patient-reported outcome (PRO) measure, because patients can complete the questionnaire themselves to provide information about their current health status and how this changes over time. ‘EQ-5D’ is not an abbreviation and is the correct term to use when referring to the instrument in general. [1] EQ-5D is widely used around the world in clinical trials and real-world clinical settings, population studies, and health economic evaluations. By mid-2020, the number of EQ-5D studies registered with the EuroQol Group totalled over 39,000. These comprised over 80 clinical areas and related to surgical procedures, hospital waiting lists, physiotherapy, general practice and primary care, and rehabilitation. The number of annual requests to use EQ-5D is approximately 5000, and EQ-5D data have been reported in over 8000 peer-reviewed papers over the past 30 years. [2] EQ-5D can be used for a variety of purposes. In clinical trials and routine clinical settings, EQ-5D can be used (i) to provide a profile of patient health on the day of questionnaire completion; (ii) to monitor the health status of patient groups at particular times, e.g. at referral, admission, discharge, and follow-up; and (iii) to measure changes in health status over time in individual patients and in cohorts of patients, such as before and after health interventions and treatments. In population studies, EQ-5D can be used to assess population health status at local and national levels and to follow population health status over time. In medical decision-making, EQ-5D can be used (i) to measure the impacts and outcomes of healthcare services; (ii) to provide relevant information for the economic evaluation of health programmes and policies; and (iii) to assist in providing evidence about effectiveness in processes where drugs or procedures require approval. EQ-5D is recommended by many health technology assessment (HTA) bodies internationally as a key component of cost-utility analyses. Kennedy-Martin M, Slaap B, Herdman M, van Reenen M, Kennedy-Martin T, Greiner W, Busschbach J, Boye KS. Which multi-attribute utility instruments are recommended for use in cost-utility analysis? A review of national health technology assessment (HTA) guidelines. Eur J Health Econ. 2020;21(8):1245-1257. doi: 10.1007/s10198-020-01195-8. PMID: 32514643; PMCID: PMC7561556. </ref> EQ-5D was developed by the EuroQol Group, and its distribution and licensing are managed by the EuroQol Research Foundation. The EuroQol website [3] provides detailed information and the latest developments about EQ-5D including guidance for users, a list of available language versions and value sets by country/region, population norms, and key EQ-5D references. It also explains how to obtain the questionnaire. Those wishing to use EQ-5D must first register their study or trial via the website, using the page ‘How to obtain EQ-5D’, [4] which has more detailed information about registering, including an animated video. EQ-5D is provided without charging a license fee to non-commercial organisations after they have registered (approximately 95% of users), while commercial users are charged a fee. Registering a study does not obligate the purchase of an EQ-5D licence, but it enables the EuroQol Research Foundation to provide further information relevant to the type of study proposed, including terms and conditions (and licence fees if applicable).
- ↑ Brooks R, Boye KS, Slaap B. EQ-5D: a plea for accurate nomenclature. J Patient Rep Outcomes. 2020;4(1):52. doi: 10.1186/s41687-020-00222-9. PMID: 32620995; PMCID: PMC7334333.
- ↑ https://pubmed.ncbi.nlm.nih.gov/?term=eq-5d&filter=dates.1990-2020%2F12%2F21 (accessed 21st December 2020).
- ↑ https://euroqol.org
- ↑ https:/euroqol.org/support/how-to-obtain-eq-5d/
2020
- https://www.physio-pedia.com/EQ-5D
- QUOTE: ... The EQ-5D is a well-established and widely-used generic instrument for assessing health-related quality of life. A cross-sectional study suggests that the EQ-5D-3L could be an excellent tool for quality of life assessment in nursing home residents with cognitive impairment[1].
- Designed as a self-completion questionnaire, it embodies two components, a health state description followed by an evaluation.
- The respondent classifies his or her prevailing state of health by selecting one of three different levels of problem severity within each of five health domains[2].
- QUOTE: ... The EQ-5D is a well-established and widely-used generic instrument for assessing health-related quality of life. A cross-sectional study suggests that the EQ-5D-3L could be an excellent tool for quality of life assessment in nursing home residents with cognitive impairment[1].
2020
- (Devlin et al., 2020) ⇒ Nancy Devlin, David Parkin, and Bas Janssen. (2020). “An Introduction to EQ-5D Instruments and their Applications.” In: Methods for Analysing and Reporting EQ-5D Data, pp. 1-22 . Springer, Cham,
- QUOTE: ... The aims of this chapter are
- to introduce the EQ-5D ‘family’ of questionnaires: what they are for, how they are used and what they measure;
- to explain the nature of the data that the EQ-5D questionnaires generate and how that affects the way that EQ-5D data should be analysed;
- to examine how the purposes for which EQ-5D data are collected affect the ways that they should be analysed and reported; and
- to describe good practice in data handling and preparing for statistical analysis of EQ-5D data.
- … The EQ-5D is ‘generic’ because it measures health in a way that can be compared across different sorts of patients, disease areas, and treatments. The researchers who developed it—the EuroQol Group—aimed to develop a questionnaire which was brief, minimised the burden of data collection, and could be used in a wide variety of health care sector applications (Devlin and Brooks 2017). The ‘5D’ in its name refers to its use of 5 dimensions for describing health states: Mobility, Usual Activities, Self-care, Pain & Discomfort and Anxiety & Depression. In the original EQ-5D questionnaire (Fig. 1.1), now known as the EQ-5D-3L, three levels of problems are described in each dimension, representing no, moderate, or extreme problems in the Pain & Discomfort and Anxiety & Depression dimensions and no, some, and inability to in the Mobility, Usual Activities and Self-care dimensions.3 In the more recent EQ-5D-5L (Fig. 1.2), the number of levels has been expanded from three to five and these are explicitly expressed as no, mild, moderate, severe and extreme or unable to (Herdman et al. 2011). A version of the instrument, the EQ-5D-Y (Fig. 1.3), has been developed for young people and children, retaining the same five dimensions (Wille et al. 2010). ...

Fig. 1.1 EQ-5D-3L descriptive system.
Source EuroQol Research Foundation. EQ-5D-3L User Guide, 2018. Latest version available from: https://euroqol.org/publications/user-guides
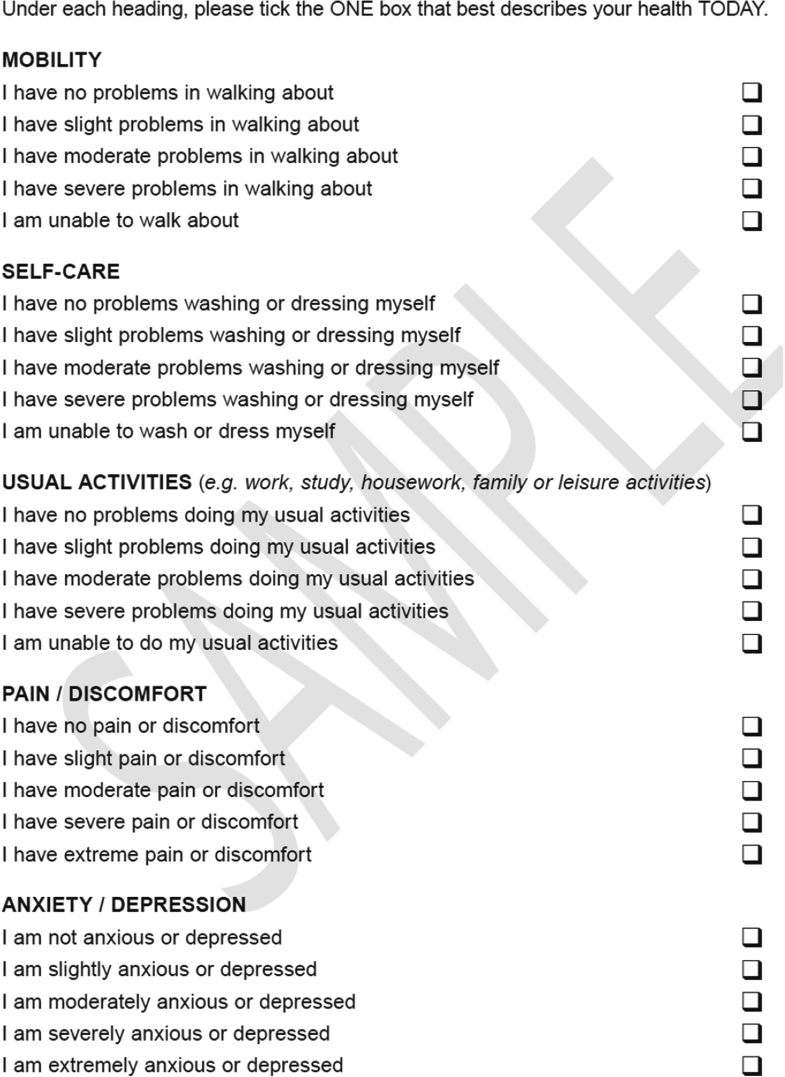
Fig. 1.2 EQ-5D-5L descriptive system.
Source EuroQol Research Foundation. EQ-5D-5L User Guide, 2019. Latest version available from: https://euroqol.org/publications/user-guides
-

Fig. 1.3 EQ-5D-Y.
Source EuroQol Research Foundation. EQ-5D-Y User Guide, 2014. Latest version available from: https://euroqol.org/publications/user-guides
- QUOTE: ... The aims of this chapter are
2017
- (Devlin & Brooks, 2017) ⇒ Nancy J. Devlin, and Richard Brooks. (2017). “EQ-5D and the EuroQol Group: Past, Present and Future.” Applied health economics and health policy 15, no. 2
- ABSTRACT: Over the period 1987–1991 an inter-disciplinary five-country group developed the EuroQol instrument, a five-dimensional three-level generic measure subsequently termed the ‘EQ-5D’. It was designed to measure and value health status. The salient features of its development and its consolidation and expansion are discussed. Initial expansion came, in particular, in the form of new language versions. Their development raised translation and semantic issues, experience with which helped feed into the design of two further instruments, the EQ-5D-5L and the youth version EQ-5D-Y. The expanded usage across clinical programmes, disease and condition areas, population surveys, patient-reported outcomes, and value sets is outlined. Valuation has been of continued relevance for the Group as this has allowed its instruments to be utilised as part of the economic appraisal of health programmes and their incorporation into health technology assessments. The future of the Group is considered in the context of: (1) its scientific strategy, (2) changes in the external environment affecting the demand for EQ-5D, and (3) a variety of issues it is facing in the context of the design of the instrument, its use in health technology assessment, and potential new uses for EQ-5D outside of clinical trials and technology appraisal.