Medical Device Development Clinical Trial
A Medical Device Development Clinical Trial is a Clinical Trial that is conducted to test and help develop a new medical device.
- AKA: Investigational Medical Device Clinical Trial, Medical Device Testing.
- Context:
- It (typically) requires regulatory approval (e.g. FDA approval via an Investigational Device Exemption Program or a Medical Device Premarket Approval Application).
- It can (typically) be smaller that a Drug Development Clinical Trial.
- It can (typically) be more difficult to blind, randomize, control and clinical endpoints highly diverse.
- It can reguire device modifications during clinical trial.
- It can (typically) be designed to support a “reasonable assurance of safety and effectiveness” for the marketing application.
- It can range from being a Medical Device Class-I Clinical Trial, to being a Medical Device Class-II Clinical Trial, to being a Medical Device Class-III Clinical Trial.
- It can range from being a Early Feasibility Clinical Study to being a First-In-Human (FIH) Clinical Trial.
- It can range from being a Medical Device (Tradicitonal) Feasibility Clinical Trial, to being Medical Device Pivotal Clinical Trial, to being a Medical Device Endpoint Clinical Trial.
- Example(s):
- NCT01943344: Safety and Performance Study of Large Hole Vascular Closure Device (FRONTIER-I),
- NCT02908880: MANTA Percutaneous Vascular Closure Device (SAFE MANTA),
- NCT03038087: A Study to Test the SENSE Device in Patients With Intracranial Hemorrhage,
- NCT03344510: Kinetic Anesthesia Device Study,
- NCT04927156: Safety, Performance and Usability of BALT Medical Devices,
- …
- Counter-Example(s):
- See: Preclinical Trial, Limited Clinical Investigation Program, Wearable Medical Device, Remote Patient Monitoring System, Decentralized Clinical Trial, Telemedicine, Digital Medicine, mHealth, Telehealth.
References
2022
- (Faris, 2022) ⇒ Owen Faris (2022). “Clinical trials for medical devices: FDA and the IDE process (PPT)". Retrived:2022-01-22.
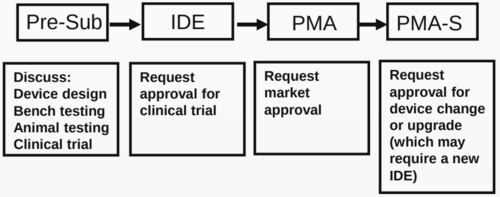
- QUOTE: Stages of review for PMA device
Device trials are unique:
- Trials tend to be smaller than drug trials.
- Some novel, many “me-too”
- Many difficult to blind, randomize, control.
- Many depend on physician technique
- Device modifications occur during trial.
- Endpoints highly diverse
- Typically, single pivotal trial follows feasibility stage(s)
- Designed to support a “reasonable assurance of safety and effectiveness” for the marketing application
- QUOTE: Stages of review for PMA device
2019
- (Wood et al., 2020) ⇒ David A. Wood, Zvonimir Krajcer, Janarthanan Sathananthan, Neil Strickman, Chris Metzger, William Fearon, Mark Aziz, Lowell F. Satler, et at. (2020). "Pivotal Clinical Study to Evaluate the Safety and Effectiveness of the MANTA Percutaneous Vascular Closure Device: The SAFE MANTA Study". In: Circulation: Cardiovascular Interventions, 12(7):e007258.DOI:10.1161/CIRCINTERVENTIONS.119.007258.
- QUOTE: The MANTA vascular closure device is a novel collagen-based technology designed to close large bore arteriotomies created by devices with an outer diameter ranging from 12F to 25F. In this study, we determined the safety and effectiveness of the MANTA vascular closure device.
Methods and Results: A prospective, single arm, multicenter investigation in patients undergoing transcatheter aortic valve replacement, percutaneous endovascular abdominal aortic aneurysm repair, or thoracic endovascular aortic aneurysm repair at 20 sites in North America. The primary outcome was time to hemostasis. The primary safety outcomes were accessed site-related vascular injury or bleeding complications. A total of 341 patients, 78 roll-in, and 263 in the primary analysis cohort, were entered in the study between November 2016 and September 2017. (...)
- QUOTE: The MANTA vascular closure device is a novel collagen-based technology designed to close large bore arteriotomies created by devices with an outer diameter ranging from 12F to 25F. In this study, we determined the safety and effectiveness of the MANTA vascular closure device.
2018
- (Byrom et al., 2018) ⇒ Bill Byrom, Marie McCarthy, Peter Schueler, and Willie Muehlhausen (2018). "Brain Monitoring Devices in Neuroscience Clinical Research: The Potential of Remote Monitoring Using Sensors, Wearables, and Mobile Devices". In: ASCPT - Clinical Pharmacology & Therapeutics, 104(1), 59-71.
- QUOTE: In clinical trials, a study endpoint is defined as a characteristic or variable that reflects how a patient feels, functions, or survives[1]. An endpoint description includes information defining how and when they are measured, how they are calculated, rules for missing data, and how they are analyzed. In the absence of formal regulatory guidance, the Critical Path Institute's ePRO Consortium reported consensus recommendations on the evidence required to support wearable device selection and endpoints derived from wearables data[2].
When using any sensor or device to measure health outcomes and endpoints in clinical research, it is important to demonstrate the reliability and validity of outcome data collected, the ability of the outcome measures to reflect one or more concepts of interest as defined by the clinical trial objectives, and to demonstrate the suitability and interpretability of endpoint measures derived from these data. Some of this evidence may be available through market clearance/certification processes, but it is not a requirement for devices to be market cleared or certified when used in clinical research.
- QUOTE: In clinical trials, a study endpoint is defined as a characteristic or variable that reflects how a patient feels, functions, or survives[1]. An endpoint description includes information defining how and when they are measured, how they are calculated, rules for missing data, and how they are analyzed. In the absence of formal regulatory guidance, the Critical Path Institute's ePRO Consortium reported consensus recommendations on the evidence required to support wearable device selection and endpoints derived from wearables data[2].
- ↑ Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 69, 89–95 (2001).
- ↑ Byrom, B. et al. Selection of and evidentiary considerations for wearable devices and their measurements for use in regulatory decision making: recommendations from the ePRO Consortium. Value Health (in press).
2017
- (Izmailova et al., 2017) ⇒ Elena S. Izmailova, John A. Wagner, and Eric D. Perakslis (2017) . "Wearable Devices in Clinical Trials: Hype and Hypothesis". DOI: 10.1002/cpt.966. In: ASCPT - Clinical Pharmology & Therapeutics.
- QUOTE: For remote monitoring of cardiovascular parameters, activity (including gait, balance, and many other forms of motion measurement), body temperature, galvanic skin response, blood oxygen saturation, and multisensor/multisystem monitoring (Majumder et al., 2017), advanced wearable device research and development is continuously improving. Common form factors include wearable watches/bracelets, patches, textiles, and garments (Table 1). All of these sensor devices are being built with the ability to monitor continuously and communicate data in real time or intermittently. While maturity, promise, and quality all vary greatly at the moment, clearly these sensors and devices have the potential to become an integral part of the future of healthcare and biopharmaceutical development.
| Device type | Data collected | Examples |
|---|---|---|
| Wrist worn | Actigraphy, HR (Heart Rate), BP (Blood Pressure), EDA (Electrodermal activity) | Actiwatch Spectrum by Phillips, ActiGraph Link by ActiGraph, E4 by Empatica, ViSi Mobile by Sotera Wireless |
| Skin patch | ECG (Electrocardiography), actigraphy, skin temperature | BioStampRC by MC10, HealthPatch by Vital Connect, BodyGuardian by Preventice |
| Cuffs | BP, HR | Intellisense Digital BP Monitor by Omron Healthcare |
| Finger worn | HR, SpO2 | iSpO2 Pulse Oximeter by Massimo |
| Clothing embedded sensors | HR, HRV (Heart Rate Variability), ECG, Breathing Rate, actigraphy | Smart shirts by Hexoskin |
| Headbands | EEG (Electroencephalogram), EMG (Electromyography) | EMOTIV EPOC by Emotiv, 4D FORCE by 4D FORCE |
2016
- (Marcus et al., 2016) ⇒ Hani J. Marcus, Christopher J. Payne, Archie Hughes-Hallett, Adam P. Marcus, Guang-Zhong Yang, Ara Darzi, and Dipankar Nandi (2016). "Regulatory approval of new medical devices: cross sectional study". In: BMJ 2016;353:i2587. DOI:10.1136/bmj.i2587.
- QUOTE: The introduction of new medical devices is fundamental to the advancement of healthcare. Historically, such devices have been adopted with little scientific evidence to support their use[1]. Although many devices have greatly improved clinical outcomes, not all are beneficial and some may be harmful. To this end most jurisdictions have developed regulatory bodies, such as the US Food and Drug Administration, that ensure the safety and effectiveness of new medical devices[2]. These regulatory bodies must also act in an efficient and timely manner such that patients are not deprived from beneficial innovations.
The process by which new high risk medical devices find their way from bench to bedside is well established: the development of the device resulting in a first-in-human study; the evaluation of the device in clinical trials, culminating in a regulatory approval for use; and the adoption of the device[3]. Although high risk devices warrant considerable scientific evidence for their safety and effectiveness before regulatory approval, the pathway for lower risk devices is less stringent, allowing for their more rapid approval[4][5][6].4 5 6
We investigated the use of these distinct regulatory approval pathways for new medical devices.
- QUOTE: The introduction of new medical devices is fundamental to the advancement of healthcare. Historically, such devices have been adopted with little scientific evidence to support their use[1]. Although many devices have greatly improved clinical outcomes, not all are beneficial and some may be harmful. To this end most jurisdictions have developed regulatory bodies, such as the US Food and Drug Administration, that ensure the safety and effectiveness of new medical devices[2]. These regulatory bodies must also act in an efficient and timely manner such that patients are not deprived from beneficial innovations.
2013
- (FDA et al., 2013) ⇒ U.S. Department of Health and Human Services Food and Drug Administration, Center for Devices and Radiological Health and Center for Biologics Evaluation and Research (2013). "Investigational Device Exemptions (IDEs) for Early Feasibility Medical Device Clinical Studies, Including Certain First in Human (FIH) Studies. Guidance for Industry and Food and Drug Administration Staff".
- QUOTE: For the purposes of this guidance, clinical study types are defined as follows:
- An early feasibility study is a limited clinical investigation of a device early in development, typically before the device design has been finalized, for a specific indication (...)
- A first in human (FIH) study is a type of study in which a device for a specific indication is evaluated for the first time in human subjects (...)
- A traditional feasibility study is a clinical investigation that is commonly used to capture preliminary safety and effectiveness information on a near-final or final device design to adequately plan an appropriate pivotal study (...)
- A pivotal study is a clinical investigation designed to collect definitive evidence of the safety and effectiveness of a device for a specified intended use, typically in a statistically justified number of subjects. It may or may not be preceded by an early and/or a traditional feasibility study(...)
- QUOTE: For the purposes of this guidance, clinical study types are defined as follows:
2004
- (Kaplan et al., 2004) ⇒ Aaron V. Kaplan, Donald S. Baim, John J. Smith, David A. Feigal, Michael Simons, David Jefferys, Thomas J. Fogarty, Richard E. Kuntz, and Martin B. Leon (2004). "Medical device development: from prototype to regulatory approval". In: Circulation, 109(25): 3068-3072. DOI: 10.1161/01.CIR.0000134695.65733.64
- QUOTE: Device development from the earliest stages requires active involvement of practicing clinicians. Clinician/inventors are frequently involved in creation of the device concept and are often integral members of the design team performing the majority of the early animal studies. Through this involvement, the clinician/inventor obtains intimate knowledge of device performance and failure modes. Safety concerns during first clinical use and pilot phase mandate participation by these clinician/inventors. The clinician/inventors frequently take leadership roles and have equity positions in the company developing the device. These interests present important conflicts of interest which must be addressed to ensure patient safety, data integrity, and public trust in the process. Many institutions have set up formal processes to address these conflicts of interest, potentially adding more time to the institutional recruitment process.
Pivotal studies required for a PMA application are typically large multicenter randomized trials and often represent the largest commercial risk and expense in the device development process. In addition to obtaining an IDE from the FDA and formally recruiting clinical sites, the sponsor must also put into place an extensive infrastructure that typically includes engaging a contract research organization (CRO), core laboratories, formation of a data safety monitoring board (DSMB), and an executive committee(...)
- QUOTE: Device development from the earliest stages requires active involvement of practicing clinicians. Clinician/inventors are frequently involved in creation of the device concept and are often integral members of the design team performing the majority of the early animal studies. Through this involvement, the clinician/inventor obtains intimate knowledge of device performance and failure modes. Safety concerns during first clinical use and pilot phase mandate participation by these clinician/inventors. The clinician/inventors frequently take leadership roles and have equity positions in the company developing the device. These interests present important conflicts of interest which must be addressed to ensure patient safety, data integrity, and public trust in the process. Many institutions have set up formal processes to address these conflicts of interest, potentially adding more time to the institutional recruitment process.
- ↑ Steiner CA, Bass EB, Talamini MA, Pitt HA, Steinberg EP. Surgical rates and operative mortality for open and laparoscopic cholecystectomy in Maryland. N Engl J Med1994;330:403-8. doi:10.1056/NEJM199402103300607 pmid:8284007.
- ↑ Barkun JS, Aronson JK, Feldman LS, et al. Balliol Collaboration. Evaluation and stages of surgical innovations. Lancet2009;374:1089-96. doi:10.1016/S0140-6736(09)61083-7 pmid:19782874.
- ↑ Drolet BC, Lorenzi NM. Translational research: understanding the continuum from bench to bedside. Transl Res2011;157:1-5. doi:10.1016/j.trsl.2010.10.002 pmid:21146144.
- ↑ Thompson M, Heneghan C, Billingsley M, Cohen D. Medical device recalls and transparency in the UK. BMJ2011;342:d2973. doi:10.1136/bmj.d2973 pmid:21572136.
- ↑ Zuckerman D, Brown P, Das A. Lack of publicly available scientific evidence on the safety and effectiveness of implanted medical devices. JAMA Intern Med2014;174:1781-7. doi:10.1001/jamainternmed.2014.4193 pmid:25265047.
- ↑ Zuckerman DM, Brown P, Nissen SE. Medical device recalls and the FDA approval process. Arch Intern Med2011;171:1006-11. doi:10.1001/archinternmed.2011.30 pmid:21321283.