Parallel Assignment Study
(Redirected from Parallel Clinical Trial)
Jump to navigation
Jump to search
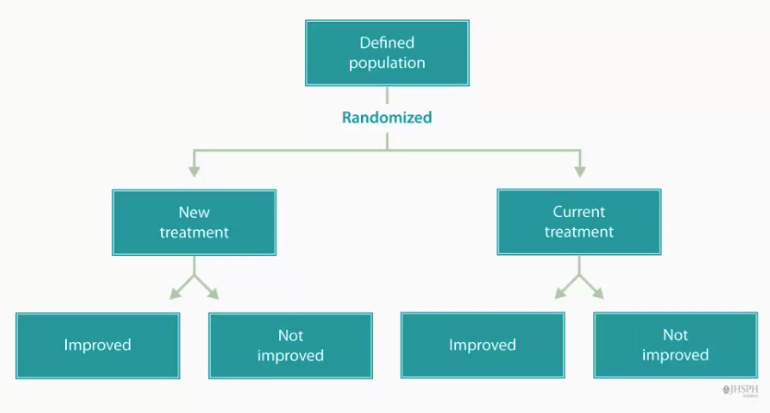
A Parallel Assignment Study is a Interventional Clinical Study in which patients' assignment and treatment administration are conducted simultaneously so that experimental and control groups are in parallel.
- AKA: Parallel Design, Parallel Group Clinical Trial.
- Context:
- It can (often) consists of assigning clinical trial participants to both treatment and control groups over the same period of time.
- It is often used to compare clinical efficacy and safety among the treatment groups.
- It typically assigns each person randomly to one treatment group.
- It can (often) adopts randomization to remove treatment selection bias and promote comparability of treatment groups.
- It can range from being a Two-Arm Parallel Clinical Trial to be an N-Arm Parallel Clinical Trial.
- Example(s):
- Counter-Example(s):
- See: Group Allocation, Randomization Unit, Clinical Trial Arm, Placebo-Controlled Clinical Trial, Equivalency Clinical Trial, Superiority Clinical Trial, Non-Inferiority Clinical Trial.
References
2022a
- (ClinicalTrials.gov, 2021) ⇒ https://clinicaltrials.gov/ct2/about-studies/glossary Retrieved 2022-01-15.
- QUOTE: Parallel assignment: A type of intervention model describing a clinical trial in which two or more groups of participants receive different interventions. For example, a two-arm parallel assignment involves two groups of participants. One group receives drug A, and the other group receives drug B. So during the trial, participants in one group receive drug A "in parallel" to participants in the other group, who receive drug B.
2022b
- (Coursera, 2021) ⇒ "Design and Interpretation of Clinical Trials" (adaptation).
- QUOTE: In a parallel design, we are assigning patients and administering treatment, so that the experimental and control groups are in parallel. In other words, we are assigning people to both groups, over the same period of time, as opposed to collecting data on the experimental groups only, and comparing that data to historical controls, or as opposed to assigning treatment A and then assigning treatment B in series.
Each person in a parallel design is assigned to only one treatment group. The process by which we allocate people to a specific treatment group is usually a randomization process. We use randomization to allocate patients because it removes bias in the allocation process, which is called selection bias.
- QUOTE: In a parallel design, we are assigning patients and administering treatment, so that the experimental and control groups are in parallel. In other words, we are assigning people to both groups, over the same period of time, as opposed to collecting data on the experimental groups only, and comparing that data to historical controls, or as opposed to assigning treatment A and then assigning treatment B in series.
|