Clinical Interventional Study Sub-Stage
(Redirected from clinical trial stage)
Jump to navigation
Jump to search
A Clinical Interventional Study Sub-Stage is a research study stage for a clinical trial process model.
- AKA: Clinical Trial Sub-Process.
- Example(s):
- Clinical Trial Protocol Design Stage.
- Clinical Trial Regulatory Review Stage.
- Clinical Trial Start-up Stage.
- Clinical Trial Site Selection and Activation/Initiation Stage.
- Clinical Trial Participant Recruitment and Enrollment Stage.
- Clinical Trial Implementation Stage.
- Clinical Trial Intervention/Data Collection Stage.
- Clinical Trial Data Analysis Stage.
- Clinical Trial Report Writing Stage.
- Clinical Trial Closeout Stage.
- Clinical Trial Followup Stage.
- Clinical Trial Dissemination of Results Stage.
- …
- See: Clinical Trials Process Model, Clinical Trial Platform.
References
2023
- chat
- Q: What are some of the main stages in a any clinical trial, regardless of phase?
- A: Regardless of the phase, clinical trials generally go through several stages, including:
- Protocol development: During this stage, the trial team develops a detailed plan for the trial, including the study objectives, design, and procedures.
- Regulatory review and approval: Before a clinical trial can begin, the trial protocol must be reviewed and approved by an ethics committee and regulatory authorities.
- Site selection and initiation: The trial team selects the locations where the trial will be conducted and prepares the sites for the trial.
- Patient recruitment: The trial team begins recruiting patients to participate in the trial.
- Data collection: During this stage, the trial team collects data from the patients according to the protocol. This may involve administering interventions, collecting blood samples, or administering questionnaires.
- Report writing and dissemination: The trial team writes a report summarizing the findings of the trial and submits the report for publication in a scientific journal.
- Follow-up: After the trial is completed, the trial team may follow up with the patients to monitor their long-term outcomes.
2023
- chat.openai.com
- Q: What are some of the typical stages in the process of delivering clinical trials?
- A: The process of delivering clinical trials typically involves a number of stages, including:
- Planning and design: This stage involves developing the study protocol, which outlines the details of the study, such as the research question being studied, the study population, the interventions being tested, and the data that will be collected. The protocol must be reviewed and approved by an ethical review board before the study can proceed.
- Site selection and activation: The research team must select the locations where the study will be conducted and ensure that the study sites are ready to begin enrolling and treating patients.
- Participant recruitment and enrollment: The research team will recruit and enroll participants for the study, following the eligibility criteria and informed consent process outlined in the protocol.
- Intervention and follow-up: During this stage, the research team will administer the interventions being tested to the study participants and collect data on their health outcomes. Follow-up visits and assessments will be conducted according to the study protocol.
- Data management and analysis: The research team will organize and analyze the data collected during the study.
- Study closeout: After the study has been completed, the research team will close out the study by completing all final study procedures, such as preparing study reports and submitting the results for publication.
- Dissemination of results: The research team will disseminate the results of the study to the scientific community and the general public through publications in scientific journals and presentations at conferences.
2020
- https://www.uab.edu/ccts/clinical-research/clinical-trials-lifecycle
- QUOTE: ... CCTS supports every stage of the clinical trial lifecycle. ...
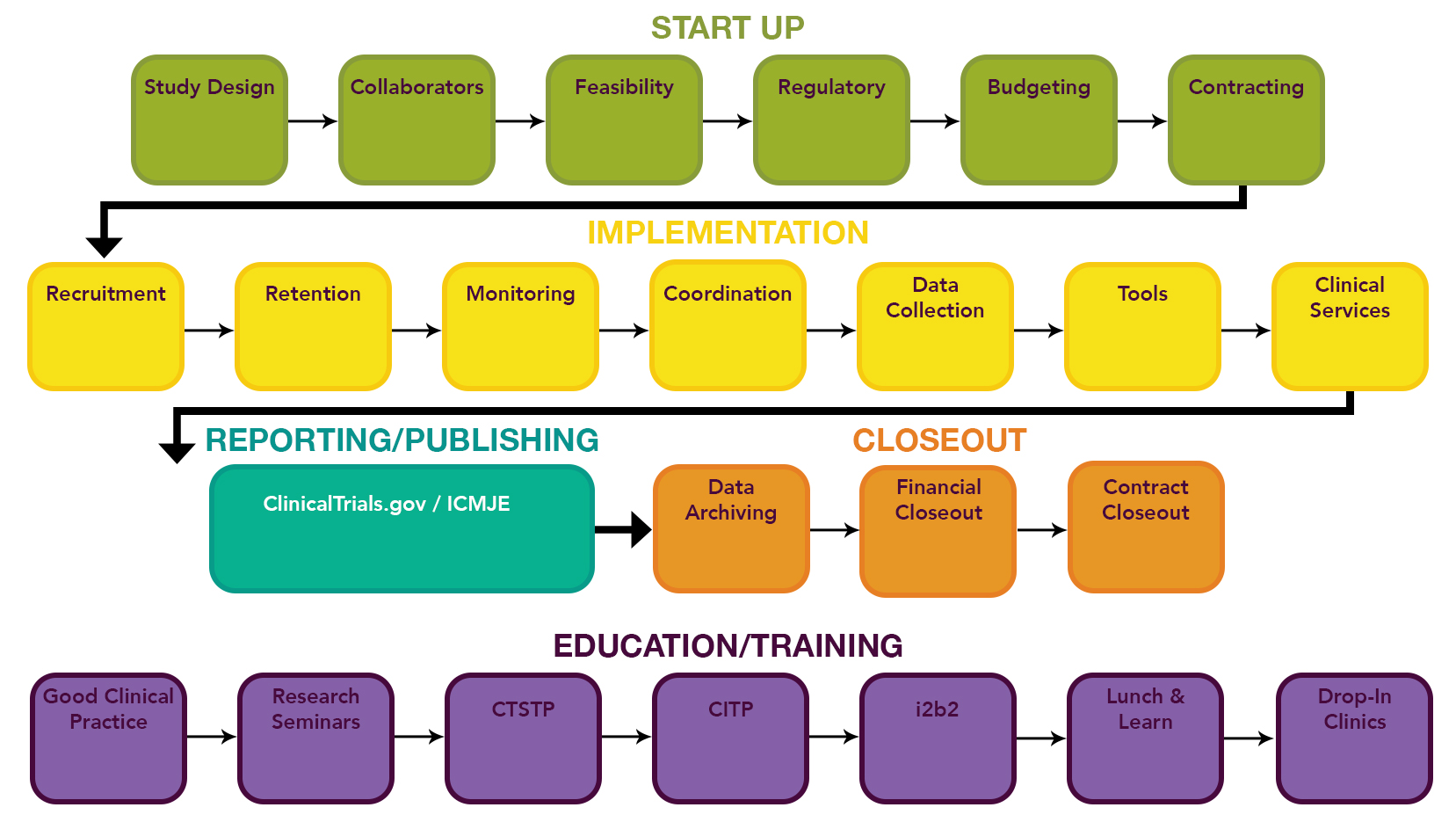
- QUOTE: ... CCTS supports every stage of the clinical trial lifecycle. ...