CDISC Therapeutic Area (TA) Standard
(Redirected from CDISC Therapeutic Standard)
Jump to navigation
Jump to search
A Clinical Data Interchange Standards Consortium Therapeutic Area (CDISC-TA) Standard is a CDISC Standard that extends the CDISC Foundational Standards to represent data that pertains to specific disease area.
- Example(s):
- Counter-Example(s):
- See: Clinical Data Interchange Standards Consortium (CDISC) RWD Connect Initiative, Standard-Developing Organization, Clinical Trial Data, Clinical Data Standards, CDISC Shared Health And Research Electronic library (SHARE), CDISC Operational Data Model (ODM), CDISC BRIDG Model. CDISC SHARE Application Programming Interface (API), CDISC SHARE Software Ecosystem.
References
2022a
- (CDISC, 2022) ⇒ https://www.cdisc.org/standards/therapeutic-areas Retrieved:2022-2-25.
- QUOTE: Therapeutic Area User Guides (TAUGs) extend the Foundational Standards to represent data that pertains to specific disease areas. TAUGs include disease-specific metadata, examples and guidance on implementing CDISC standards for a variety of uses, including global regulatory submissions.
2022b
- (Facile et al., 2022) ⇒ Rhonda Facile, Erin Elizabeth Muhlbradt, Mengchun Gong, Qingna Li, Vaishali Popat, Frank Petavy, Ronald Cornet, Yaoping Ruan, Daisuke Koide, Toshiki I. Saito, Sam Hume, Frank Rockhold, Wenjun Bao, Sue Dubman, Barbara Jauregui Wurst (2022). "Use of Clinical Data Interchange Standards Consortium (CDISC) Standards for Real-world Data: Expert Perspectives From a Qualitative Delphi Survey". In: JMIR medical informatics, 10(1), e30363.
- QUOTE: The CDISC standards span the clinical research process and include standards for the exchange of nonclinical data (SEND), data collection case report forms (CRFs; clinical data acquisition standards harmonization (CDASH)), aggregation and tabulation (study data tabulation model (SDTM)), Biomedical Research Integrated Domain Group (BRIDG) logical model, and operational data model (ODM) for transport (Figure 1). In collaboration with the National Cancer Institute's Enterprise Vocabulary Services (NCI-EVS) program, CDISC has developed a rich controlled terminology that is linked to other common research semantics through the NCI-EVS tools. These standards, presented in data models, implementation guides, and user guides, are globally recognized and heavily used by the biopharmaceutical industry and some academic institutions.
2018
- (Hume et al., 2018) ⇒ Samuel Hume, Anthony Chow, Julie Evans, Frederik Malfait, Julie Chason, J. Darcy Wold, Wayne Kubick,and Lauren B. Becnel (2018). "CDISC SHARE, a Global, Cloud-based Resource of Machine-Readable CDISC Standards for Clinical and Translational Research". In: AMIA Summits on Translational Science Proceedings, 2018, 94.
- QUOTE: CDISC standards (Figure 1) are categorized as Foundational Standards, Semantics, Therapeutic Area (TA) Standards, and Transport Standards (...)
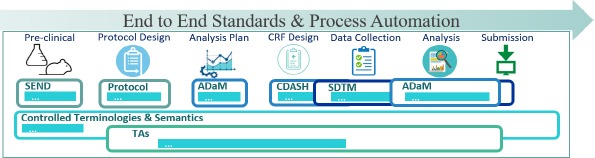
|
- Except for Protocol, the foundational standards and their implementation guides are organized into classes of related categories of information. Within SDTM for example, each class is subdivided into domains, and each domain is comprised of variables. Semantics include CDISC Controlled Terminologies, developed in collaboration with NCI EVS, and the Biomedical Research Integrated Domains Group (BRIDG) domain information model. TA User Guides provide indication-specific “slices” of foundational standards and their terminologies, with examples of how to use these standards for a given TA. Transport Standards support submissions, data interchange, study archival, and automated study setup[1].
