Clinical Study Report (CSR)
Jump to navigation
Jump to search
A Clinical Study Report (CSR) is a report document that provides the information of the methods and results of a clinical study.
- Context:
- It can be associated with a CSR Synopsis.
- …
- Example(s):
- an NCT01720524 CSR for ... [1].
- an NCT00806026 CSR for a pregabalin PHASE III Trial (from 2008-12-23 to 2011-04-28) [2].
- an NCT00038103 CSR for a celecoxib PHASE II Trial by Pfizer (from 2002-01-23 to 2008-03-27) [3].
- an NCT00847613 CSR for a tofacitinib PHASE III Trials by Pfizer (from 2009-03-31 to 2012-02-10) [4]
- …
- Counter-Example(s):
- See: Clinical Trial Publication.
References
2022
- (Wikipedia, 2022) ⇒ https://en.wikipedia.org/wiki/clinical_study_report Retrieved:2022-01-12.
- In medicine, a clinical study report (CSR) on a clinical trial is a document, typically very long, providing much detail about the methods and results of a trial. A CSR is a scientific document addressing efficacy and safety, not a sales or marketing tool; its content is similar to that of a peer-reviewed academic paper. [1] Results of trials are usually reported in a briefer academic journal paper, but methodological flaws are often glossed over in the briefer paper.[2] The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) is a body bringing together the regulatory authorities and pharmaceutical industry of Europe, Japan and the US to discuss scientific and technical aspects of drug registration; [3] in 1995 it produced a tripartite harmonised ICH guideline on the format and content of a study report to be acceptable in all three ICH regions. [4] Recommended prerequisites and content for producing a report conformant to ICH guidelines have been outlined by SE Caldwell. [5] In the Nov 9, 2016 addendum to the ICH guidelines Canada and Switzerland were added to the countries which would accept the unified standard.
- ↑ Bellevue Biomedical: Writing your first clinical study report
- ↑ Ben Goldacre, Statins have no side effects? This is what our study really found …, Guardian newspaper, 15 March 2014
- ↑ ICH Web site
- ↑ ICH: Structure and content of clinical study reports E3
- ↑ Things Medical Writers Need for Clinical Study Reports (CSRs)
2021
- https://pfizer.com/science/clinical-trials/data-and-results/trial-results
- QUOTE: ... Clinical Study Reports (CSRs) are often created as part of the process of submitting applications for new medical treatments to regulators. CSRs answer questions such as: Why was the trial done? What were the important questions asked in the trial? What were the results? CSRRs also include extensive details on the course of treatment for patients, the medical information collected from the patients as part of the research, and demographic data, as well as other kinds of information to explain how the trial was conducted and results were analyzed.
Because CSRs contain information that could be valuable to researchers, Pfizer is making electronic synopses of CSRs publicly available on this website. They include the synopsis of the CSR submitted to the regulatory agency. ...
- QUOTE: ... Clinical Study Reports (CSRs) are often created as part of the process of submitting applications for new medical treatments to regulators. CSRs answer questions such as: Why was the trial done? What were the important questions asked in the trial? What were the results? CSRRs also include extensive details on the course of treatment for patients, the medical information collected from the patients as part of the research, and demographic data, as well as other kinds of information to explain how the trial was conducted and results were analyzed.
2021
- https://www.narrativa.com/automation-of-clinical-study-reports-csr-using-artificial-intelligence/
- QUOTE: ... Medical writers spend a large amount of time creating Clinical Study Reports (CSRs), a time-consuming undertaking that could be better spent on higher-value tasks. Narrativa offers Natural Language Generation (NLG) using advanced artificial intelligence (AI) to transform clinical trial data into Clinical Study Reports. ...
...
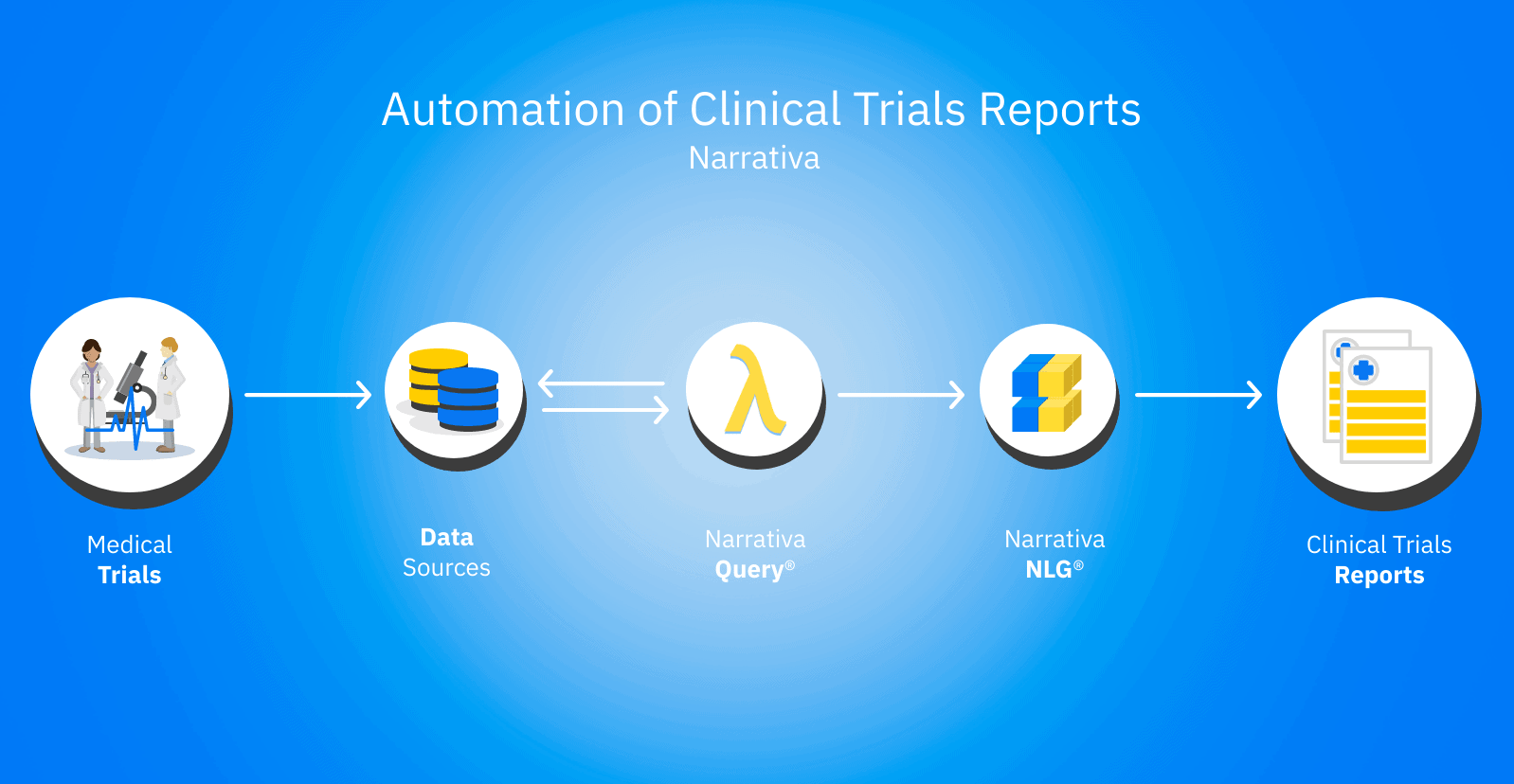
- QUOTE: ... Medical writers spend a large amount of time creating Clinical Study Reports (CSRs), a time-consuming undertaking that could be better spent on higher-value tasks. Narrativa offers Natural Language Generation (NLG) using advanced artificial intelligence (AI) to transform clinical trial data into Clinical Study Reports. ...