Crossover Clinical Trial
A Crossover Clinical Trial is a clinical trial that is a crossover experiment (where each patient receives a sequence of different treatments, but the order in which treatments are administrated is randomized).
- Context:
- It can have advantages, such as:
- each patient serves as his/her own control group;
- variability is reduced because there is less variability within patients than between patients;
- fewer patients needed.
- It can have disadvantages, such as:
- the conditions, for which crossover design can be used, are only those that have a chronic level of intensity for which the treatments provide symptomatic relief but not any permanent cure or effects.
- Some effects from a treatment in any early period will potentially carry over to later periods. This implies that the washout period has to be long enough to accommodate the treatment that has the longest potential carryover effect.
- Dropout rate can be higher than other trials as crossover trial are usually of longer duration. Dropouts can be more problematic as it usually involves a fewer patients than other trial.
- Data analysis can be more complex than a parallel design, because correlated outcomes on the individual has to be raken into account.
- …
- It can have advantages, such as:
- Example(s):
- NCT03068312 (VX16-770-127): Crossover Clinical Trial- Ivacaftor (Cystic Fibrosis).
- NCT00444184: 24-hour Intraocular Pressure Control With Travoprost/Timolol Fixed Combination Versus Travoprost,
- NCT00127166: Crossover clinical trial - Montelukast vs Salmeterol (exercise-induced asthma in children),
- …
- Counter-Example(s):
- See: Washout Period, Carry-Over Effect, Clinical Trial Dropout, Group Allocation, Randomization Unit, Clinical Trial Arm, Placebo-Controlled Clinical Trial, Equivalency Clinical Trial, Superiority Clinical Trial, Non-Inferiority Clinical Trial.
References
2022a
- (ClinicalTrials.gov, 2021) ⇒ https://clinicaltrials.gov/ct2/about-studies/glossary Retrieved 2022-01-15.
- QUOTE: Cross-over assignment: A type of intervention model describing a clinical trial in which groups of participants receive two or more interventions in a specific order. For example, two-by-two cross-over assignment involves two groups of participants. One group receives drug A during the initial phase of the trial, followed by drug B during a later phase. The other group receives drug B during the initial phase, followed by drug A. So during the trial, participants "cross over" to the other drug. All participants receive drug A and drug B at some point during the trial but in a different order, depending on the group to which they are assigned.
2022b
- (Coursera, 2021) ⇒ "Design and Interpretation of Clinical Trials" (adaptation).
- QUOTE: In a crossover design, the unit that is randomized is the order in which the treatments are received, instead of whether the patient receives treatment A or B. In other words, we randomize whether a patient receives treatment A first and then B, or B and then A.
Randomization promotes balance between the treatment groups and time of the exposure. The defining feature of our crossover design is that we are testing each treatment in all patients. This means that each patient serves as his or her own control group. This is a nice feature because variability is often higher between measurements of an outcome taken on different people, than in repeated measurements taken on the same person. If each person serves his or her own control group, we are essentially controlling for other person-level characteristics that may affect the outcome measurement and increase variability. An advantage of the reduction in variability is that we need fewer patients to test the hypothesis of interest.
Crossover design is more efficient than the comparable parallel design.
- QUOTE: In a crossover design, the unit that is randomized is the order in which the treatments are received, instead of whether the patient receives treatment A or B. In other words, we randomize whether a patient receives treatment A first and then B, or B and then A.
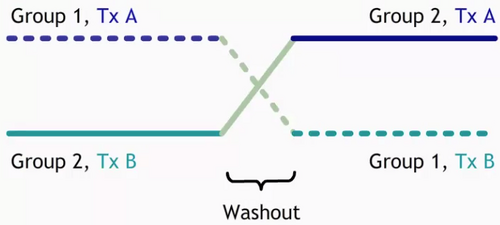
- Fig. 2 is a graphic representation of crossover trial, group 1 is represented by a dashed line and group 2 is represented by a solid line. On the graph’s top left corner, we see that group 1 receives treatment A first, followed by a washout period that is represented here with a green line, and then group 1 receives treatment B. Group 2 receives treatment B first, followed by a washout period, and then group 2 receives treatment A.
2022c
- (Wikipedia, 2022) ⇒ https://en.wikipedia.org/wiki/Crossover_study Retrieved:2022-1-16.
- In medicine, a crossover study or crossover trial is a longitudinal study in which subjects receive a sequence of different treatments (or exposures). While crossover studies can be observational studies, many important crossover studies are controlled experiments, which are discussed in this article. Crossover designs are common for experiments in many scientific disciplines, for example psychology, pharmaceutical science, and medicine.
Randomized, controlled crossover experiments are especially important in health care. In a randomized clinical trial, the subjects are randomly assigned to different arms of the study which receive different treatments. When the trial has a repeated measures design, the same measures are collected multiple times for each subject. A crossover trial has a repeated measures design in which each patient is assigned to a sequence of two or more treatments, of which one may be a standard treatment or a placebo.
Nearly all crossover are designed to have "balance", whereby all subjects receive the same number of treatments and participate for the same number of periods. In most crossover trials each subject receives all treatments, in a random order.
Statisticians suggest that designs should have four periods, which is more efficient than the two-period design, even if the study must be truncated to three periods. Vonesh & Chinchilli (1997) Jones & Kenward (2003) However, the two-period design is often taught in non-statistical textbooks, partly because of its simplicity.
- In medicine, a crossover study or crossover trial is a longitudinal study in which subjects receive a sequence of different treatments (or exposures). While crossover studies can be observational studies, many important crossover studies are controlled experiments, which are discussed in this article. Crossover designs are common for experiments in many scientific disciplines, for example psychology, pharmaceutical science, and medicine.
2021
- (Kerem et al., 2021) ⇒ Eitan Kerem, Malena Cohen-Cymberknoh, Reuven Tsabari, Michael Wilschanski, Joel Reiter, David Shoseyov, Alex Gileles-Hillel, Thea Pugatsch, Jane C. Davies, Christopher Short, Clare Saunders, Cynthia DeSouza, James C. Sullivan, Jamie R. Doyle, Keval Chandarana, and Nils Kinnman (2021). "Ivacaftor in people with cystic fibrosis and a 3849+ 10kb C→ T or D1152H residual function mutation". In: Annals of the American Thoracic Society, 18(3), 433-441.
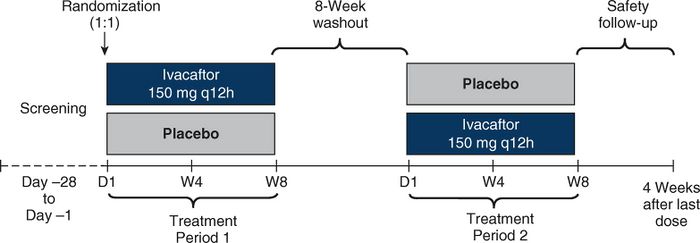
- QUOTE: This was a phase 3b, randomized, double-blind, placebo-controlled, single-center crossover study in people with CF $geq 6$ years of age with a $3849 + 10kb\; C\to T$ or D1152H CFTR gene mutation and percentage predicted forced expiratory volume in 1 second (ppFEV1) of $geq 40\%$ and $\leq105\%$ at screening (NCT03068312). Participants were randomized (1:1) to one of two sequences (ivacaftor→ placebo or placebo→ivacaftor) that included two 8-week treatment periods with ivacaftor (150 mg every 12 h) or matching placebo, separated by an 8-week washout period (Figure 1).
2011
- (Evans, 2011) ⇒ Scott R. Evans (2011). "Clinical Trial Structures". In: Journal of Experimental Stroke & Translational Medicine, 3(1):8-18.
- QUOTE: In a crossover design, each participant is randomized to a sequence of treatments that will be sequentially administered during treatment periods although the objective remains a comparison of treatments. For instance, in a two-period, two-sequence (2 × 2) crossover trial designed to compare two treatments A and B, a participant is randomized into one of two sequences: (1) A then B, or (2) B then A. The randomization of the treatment sequence helps to account for temporal trends (such as seasonal variation).
Crossover trials have several advantages. Firstly, they generally require fewer participants than parallel designs because each participant serves as his/her own control, thus eliminating inter-participant variation. A crossover study may reduce the sample size of a parallel group study by 60–70% in some cases. Also since each participant is evaluated for each treatment, potentially confounding variables are balanced between treatment groups by design, hence making treatment comparisons “fair”. Secondly, researchers can study individual participant response to treatment and examine participant-by-treatment interactions. Lastly, study recruitment may be enhanced as potential participants are aware that they will receive active treatment at some point during the study.
However crossover trials should be used selectively. The primary concern with crossover trials is the potential “carry-over effect”. If the residual effect of the treatment provided in the first period continues into the second period when assessments of the second treatment are made (despite the discontinuation of the treatment at the end of the first period), then treatment comparisons could be biased since one cannot distinguish between the treatment effect and the carry-over effect. For this reason, a “washout” period is often built into the study design to separate two treatment periods to eliminate “carry-over” effects. A frequent recommendation is for the washout period to be at least 5 times the half-life of the treatment with the maximum half-life in the study. Endpoint evaluations can also be made at the end of a period to allow more time for the effects of prior treatments to dissipate. A second concern with crossover trials is the increased rate of participant drop-out. The drop-outs rate may be high in a crossover study since the trials are generally longer in duration for each participant, to accommodate for multiple treatment periods and associated washout periods. Participants are also exposed to more potentially harmful treatments and thus may be more likely to drop-out due to toxicity. The ramification of drop-outs in a crossover study is a threat to the generalizability of the study results as analyses are generally conducted on only the subset of participants that completed at least two periods.
- QUOTE: In a crossover design, each participant is randomized to a sequence of treatments that will be sequentially administered during treatment periods although the objective remains a comparison of treatments. For instance, in a two-period, two-sequence (2 × 2) crossover trial designed to compare two treatments A and B, a participant is randomized into one of two sequences: (1) A then B, or (2) B then A. The randomization of the treatment sequence helps to account for temporal trends (such as seasonal variation).