Equivalence Clinical Trial
An Equivalence Clinical Trial is a Clinical Trial which primary objective is to show that participants' response to two or more treatment are essentially equivalent (i.e. clinically similar).
- AKA: Equivalency Clinical Trial.
- Context:
- It usually measures an equivalence margin, $\delta$, which helps determine the clinical similarity between treatments.
- Example(s):
- Counter-Example(s):
- See: Randomized Clinical Trial, Clinical Trial Participant, Clinical Trial, Phase 1 Clinical Trial, Phase 2 Clinical Trial, Phase 3 Clinical Trial, Placebo-Controlled Clinical Trial, Drug Development Clinical Trial, Double-Blinded Clinical Trial.
References
2021
- (Wikipedia, 2021) ⇒ https://en.wikipedia.org/wiki/Glossary_of_clinical_research#E Retrieved:2021-12-26.
- QUOTE: Equivalence trial
- A trial with the primary objective of showing that the response to two or more treatments differs by an amount which is clinically unimportant. This is usually demonstrated by showing that the true treatment difference is likely to lie between a lower and an upper equivalence margin of clinically acceptable differences. (ICH E9)
- QUOTE: Equivalence trial
2017
- (Wang et al., 2017) ⇒ Bokai Wang, Hongyue Wang, Xin M. Tu, and Changyong Feng (2017) "Comparisons of Superiority, Non-inferiority, and Equivalence Trials". In: Shanhai Archives of Psychiatry, 29(6). DOI:10.11919/j.issn.1002-0829.217163.
- QUOTE: In a superiority trial, we want to show that the new treatment intervention (drug, psychotherapy) is superior to (better than) the control condition. For example, we want to know if a new drug can significantly increase CD4 counts for HIV patients or a novel psychosocial therapy will increase social activities for lonely old adults(...)
A non-inferiority trial is to show that treatment A is not worse than the treatment B. Although these kinds of trials are not used to establish better treatment efficacy, the new method may have advantages over current methods in other aspects. For example, the new intervention may be less costly, less invasive, and have less side effects.
The hypotheses of non-inferiority clinical trials are
- QUOTE: In a superiority trial, we want to show that the new treatment intervention (drug, psychotherapy) is superior to (better than) the control condition. For example, we want to know if a new drug can significantly increase CD4 counts for HIV patients or a novel psychosocial therapy will increase social activities for lonely old adults(...)
| $H_0:\mu_1-\mu_0\leq-\delta$ vs. $H_1:\mu_1-\mu_0>-\delta$ | (5) |
- where $\delta \geq 0$ and is also called the margin of clinical significance which is usually small(...)
"Equivalence” does not mean “equal” or "same" as in practice. When we say the treatment and the control are equivalent, we mean that they are "similar". By quantifying “Similarity” using a tolerance range, the hypotheses for an equivalence trial are specified as
- where $\delta \geq 0$ and is also called the margin of clinical significance which is usually small(...)
|
$H_0:|\mu_1-\mu_0|\geq\delta$ vs. $H_1:|\mu_1-\mu_0|<\delta$ |
(6) |
- where $\delta > 0$ is a pre-specified tolerance margin. If the null hypothesis is rejected, then the mean difference of two groups is within the tolerance range and the treatment and control are equivalent.
A closer look at (6) shows the hypotheses in an equivalence trial are the same as
- where $\delta > 0$ is a pre-specified tolerance margin. If the null hypothesis is rejected, then the mean difference of two groups is within the tolerance range and the treatment and control are equivalent.
| $H_0:\mu_1-\mu_0\leq-\delta$ and $\mu_0-\mu_1\leq -\delta$ vs. $H_1:\mu_1-\mu_0>\delta$ and $\mu_0-mu_1\geq>-\delta$ | (6) |
- Comparing (5) and (6) we can see that the equivalence trial is the intersection of two non-inferiority trials. Intuitively, the treatment and control are equivalent, if and only if neither one is inferior to the other.
2015
- (Cohen et al., 2015) ⇒ Jeffrey Cohen, Anna Belova, Krzysztof Selmaj, Christian Wolf, Maria Pia Sormani, Janine Oberye, Evelyn van den Tweel, Roel Mulder, Norbert Koper, Gerrit Voortman, Frederik Barkhof, and for the Glatiramer Acetate Clinical Trial to Assess Equivalence With Copaxone (GATE) Study Group. (2015). “Equivalence of Generic Glatiramer Acetate in Multiple Sclerosis: A Randomized Clinical Trial.” In: JAMA Neurology, 72(12).
- QUOTE: The GATE study, to our knowledge, is the first phase 3 clinical trial to date of a generic disease-modifying medication for MS. The patents for the first approved treatments for RRMS are expiring, creating the opportunity to develop generic alternatives, with the goal of cost savings for payers and patients. The development of generic glatiramer acetate illustrates the challenges in developing generic biological and complex nonbiological agents. The GATE study demonstrated equivalent efficacy, safety, and tolerability for generic glatiramer acetate and brand glatiramer acetate as treatment for RRMS. These results may allow for a generic alternative to the originator brand glatiramer acetate, an RRMS treatment with established long-term efficacy and safety.
2011
- (Walker & Nowacki, 2011) ⇒ Esteban Walker, and Amy S. Nowacki (2011) "Understanding Equivalence and Noninferiority Testing". In: Springer - Journal of General Internal Medicine, 26(2). DOI:10.1007/s11606-010-1513-8.
- QUOTE: The determination of the equivalence margin, $\delta$, is the most critical step in equivalence/noninferiority testing. A small value of $\delta$ determines a narrower equivalence region, and makes it more difficult to establish equivalence/noninferiority. The equivalence margin not only determines the result of the test, but also gives scientific credibility to a study. The value and impact of a study depend on how well the equivalence margin can be justified in terms of relevant evidence and sound clinical considerations. Frequently, regulatory issues also have to be considered.[1]
An equivalence/noninferiority study should be designed to minimize the possibility that a new therapy that is found to be equivalent/noninferior to the current therapy can be nonsuperior to a placebo. One way to minimize this possibility is to choose a value of the equivalence margin based on the margin of superiority of the current therapy against the placebo. This margin of superiority can be estimated from previous studies. In noninferiority testing, a common practice is to set the value of $\delta$ to a fraction, $f$, of the lower limit of a confidence interval of the difference between the current therapy and the placebo obtained from a meta-analysis. The smaller the value of $f$, the more difficult the establishment of equivalence/noninferiority of the new therapy.
- QUOTE: The determination of the equivalence margin, $\delta$, is the most critical step in equivalence/noninferiority testing. A small value of $\delta$ determines a narrower equivalence region, and makes it more difficult to establish equivalence/noninferiority. The equivalence margin not only determines the result of the test, but also gives scientific credibility to a study. The value and impact of a study depend on how well the equivalence margin can be justified in terms of relevant evidence and sound clinical considerations. Frequently, regulatory issues also have to be considered.[1]
- ↑ Garret AD. Therapeutic equivalence: fallacies and falsification. Statist Med. 2003;22:741–762. doi: 10.1002/sim.1360.
2009
- (Greene et al., 2009) ⇒ Carolyn J. Greene, Leslie A. Morland, Valerie L. Durkalski, and B. Christopher Frueh (2009). "Noninferiority and Equivalence Designs: Issues and Implications for Mental Health Research". In: Journal of Traumatic Stress - ISTSS Willey, 21(5). DOI:10.1002/jts.20367.
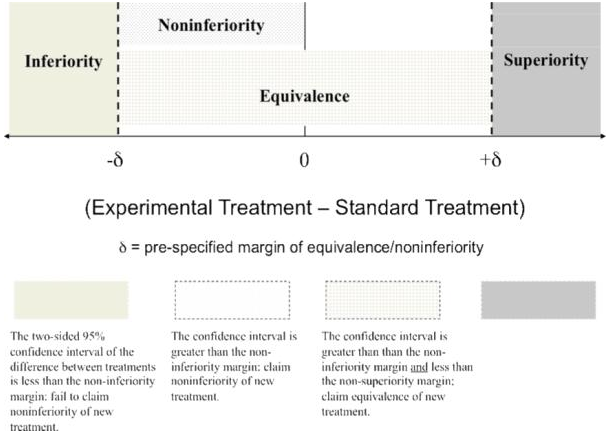
- QUOTE: Noninferiority and equivalence designs are becoming increasingly common and relevant in randomized controlled trials (RCTs) in the fields of medicine and mental health. Noninferiority designs are a one-sided test used to determine if a novel intervention is no worse than a standard intervention. Equivalence designs, a two-sided test, pose a similar question, but also allow for the possibility that the novel intervention is no better than the standard one. Issues related to the comparability between novel interventions and better established ones are of great importance to improving health service delivery across a wide range of settings and contexts. However, noninferiority and equivalence designs are often poorly understood, improperly applied, and subsequently misinterpreted. These designs differ from traditional “superiority” trials in significant conceptual and statistical ways and pose a unique set of challenges to investigators and consumers of research(...)
Figure 1 provides an illustration of this zone. Equivalency designs are rarely used in therapeutic trials evaluating effectiveness since the study objective often is to show that a new treatment is not inferior to a standard, which corresponds to a noninferiority design, as opposed to showing that the novel treatment is neither inferior nor superior to the standard, which corresponds to the equivalency design. Because noninferiority trials are much more common in mental health research, this article focuses on that design.
- QUOTE: Noninferiority and equivalence designs are becoming increasingly common and relevant in randomized controlled trials (RCTs) in the fields of medicine and mental health. Noninferiority designs are a one-sided test used to determine if a novel intervention is no worse than a standard intervention. Equivalence designs, a two-sided test, pose a similar question, but also allow for the possibility that the novel intervention is no better than the standard one. Issues related to the comparability between novel interventions and better established ones are of great importance to improving health service delivery across a wide range of settings and contexts. However, noninferiority and equivalence designs are often poorly understood, improperly applied, and subsequently misinterpreted. These designs differ from traditional “superiority” trials in significant conceptual and statistical ways and pose a unique set of challenges to investigators and consumers of research(...)
|