Investigational Medical Device
Jump to navigation
Jump to search
An Investigational Medical Device is a Medical Device that has been approved by a government or regulatory authority to be tested in an investigational clinical trial.
- Example(s):
- Counter-Example(s):
- See: Investigational Treatment, Institutional Review Board (IRB) Approval, Medical Device Regulation (MDR), Investigational Device Exemption (IDE) Program, Medical Device Development Clinical Trial, Medical Device Premarket Approval Application (PMA).
References
2022a
- (FDA, 2022) ⇒ https://www.fda.gov/medical-devices/device-advice-comprehensive-regulatory-assistance/overview-device-regulation Retrieved:2022-3-20.
- QUOTE: FDA's Center for Devices and Radiological Health (CDRH) is responsible for regulating firms who manufacture, repackage, relabel, and/or import medical devices sold in the United States. In addition, CDRH regulates radiation-emitting electronic products (medical and non-medical) such as lasers, x-ray systems, ultrasound equipment, microwave ovens and color televisions.
- Medical devices are classified into Class I, II, and III. Regulatory control increases from Class I to Class III. The device classification regulation defines the regulatory requirements for a general device type. Most Class I devices are exempt from Premarket Notification 510(k); most Class II devices require Premarket Notification 510(k); and most Class III devices require Premarket Approval (...)
2022b
- (Wikipedia, 2022) ⇒ https://en.wikipedia.org/wiki/Glossary_of_clinical_research#I Retrieved:2022-3-20.
- Investigational
- In clinical trials, refers to a drug (including a new drug, dose, combination, or route of administration) or procedure that has undergone basic laboratory testing and received approval from the U.S. Food and Drug Administration (FDA) to be tested in human subjects. A drug or procedure may be approved by the FDA for use in one disease or condition, but be considered investigational in other diseases or conditions. Also called experimental. (NCI)
- Investigational
2022c
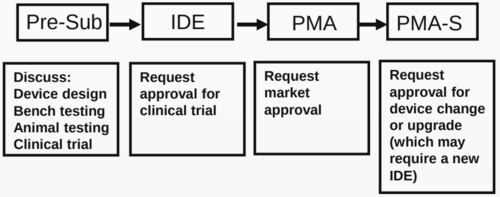
- (Faris, 2022) ⇒ Owen Faris (2022). “Clinical trials for medical devices: FDA and the IDE process (PPT)". Retrived:2022-01-22.
- QUOTE: Stages of review for PMA device
Device trials are unique:
- Trials tend to be smaller than drug trials.
- Some novel, many “me-too”
- Many difficult to blind, randomize, control.
- Many depend on physician technique
- Device modifications occur during trial.
- Endpoints highly diverse
- Typically, single pivotal trial follows feasibility stage(s)
- Designed to support a “reasonable assurance of safety and effectiveness” for the marketing application.
- QUOTE: Stages of review for PMA device