Interventional Clinical Study Process Model
(Redirected from Clinical Trial Lifecycle)
Jump to navigation
Jump to search
A Interventional Clinical Study Process Model is a clinical study process model for a clinical trial process.
- Context:
- It can (often) reference Clinical Trial Process Stages, such as:
- CT Protocol Development Stage: This stage involves the creation of a detailed plan for the clinical trial, including the study design, objectives, eligibility criteria, and outcome measures. This plan must be approved by an ethics committee before the trial can proceed.
- CT Site Selection and Initiation Stage: In this stage, the clinical trial sponsor selects the locations where the trial will be conducted and establishes relationships with the sites and investigators.
- CT Participant Recruitment and Screening Stage: This stage involves recruiting eligible participants and screening them to ensure they meet the criteria for participating in the trial. This may include a review of medical records and conducting physical exams and laboratory tests.
- CT Study Execution Stage: During this stage, the study is carried out according to the protocol. Participants are randomly assigned to receive either the experimental treatment or a control treatment, and their outcomes are monitored and recorded.
- CT Monitoring Stage: During this stage, an independent monitoring committee or a clinical research organization may be responsible for monitoring the progress of the trial, ensuring that it is conducted according to the protocol and Good Clinical Practice (GCP) guidelines, and ensuring the safety and welfare of the participants.
- CT Database Cleaning and Lock: This stage involves cleaning and verifying the data collected during the study to ensure its accuracy and completeness. Once this process is complete, the database is locked to prevent any further changes.
- CT Auditing Stage: In this stage, an independent auditor may review the trial data and procedures to confirm their accuracy and compliance with GCP guidelines.
- CT Amendment Stage: This stage may be necessary if changes need to be made to the protocol during the course of the trial. This could be due to unexpected safety concerns, changes in regulatory requirements, or other factors.
- CT Data Safety Monitoring Board (DSMB) Review: In some trials, a Data Safety Monitoring Board (DSMB) may be established to review data from the trial on a regular basis to ensure the safety of the participants and the validity of the results.
- CT Analysis and Reporting: In this stage, the data collected during the study is analyzed to determine the efficacy and safety of the experimental treatment. The results are then reported in scientific journals and presented at conferences.
- CT Study Closeout: This is the final stage of a clinical trial, during which the trial is officially ended, and all necessary follow-up evaluations and reporting are completed. Any remaining study materials and equipment are returned or disposed of in accordance with applicable regulations.
- ...
- It can combine Clinical Trial User Journey Models, such as a clinical trial patient journey model, clinical trial site journey model, and/or a clinical trial sponsor journey model.
- …
- It can (often) reference Clinical Trial Process Stages, such as:
- Example(s):
- the one followed by …
- ...
- Counter-Example(s):
- See: Patient Perspective, Clinical Trial Sponsor Perspective.
References
https://www.appliedclinicaltrialsonline.com/view/ctms-what-you-should-know https://www.clinicaltrialsarena.com/news/operational-excellence-in-clinical-trials-4853862-2/
2020
- https://www.uab.edu/ccts/clinical-research/clinical-trials-lifecycle
- QUOTE: ... CCTS supports every stage of the clinical trial lifecycle. ...
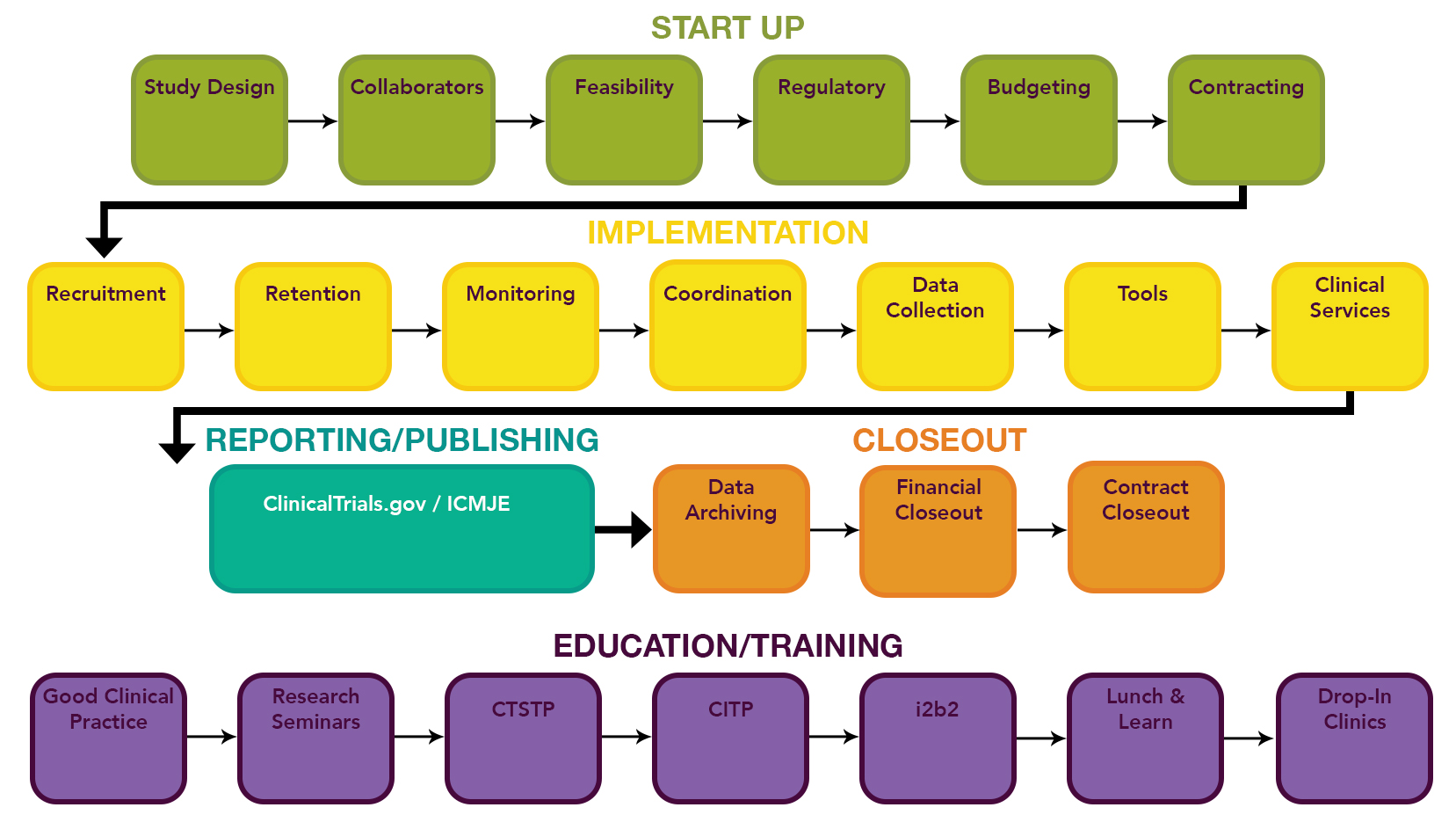
- QUOTE: ... CCTS supports every stage of the clinical trial lifecycle. ...
2018
- (Sommer et al., 2018) ⇒ Carsten Sommer, Diego Zuccolin, Valdo Arnera, Nicole Schmitz, Pernilla Adolfsson, Nicoletta Colombo, Raphaelle Gilg, and Bryan McDowell. (2018). “Building Clinical Trials Around Patients: Evaluation and Comparison of Decentralized and Conventional Site Models in Patients with Low Back Pain.” In: Contemporary Clinical Trials Communications, 11. https://doi.org/10.1016/j.conctc.2018.06.008
- QUOTE:

- QUOTE:
2017
- (2017) "Understand Clinical Trials Changes at the NIH."
- QUOTE:
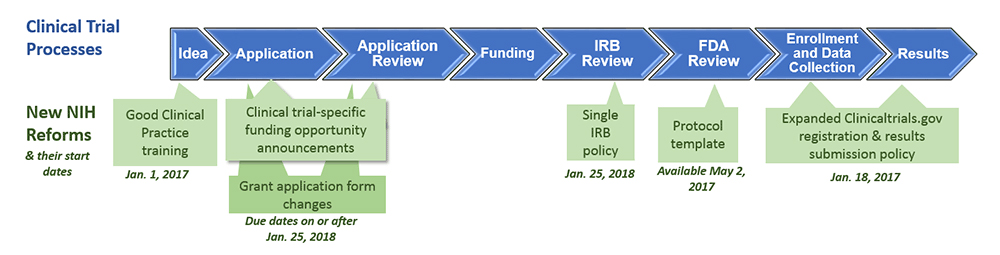
- QUOTE:
2016
- (Dagalur, 2016) ⇒ Srini Dagalur. (2016). “CTMS: What You Should Know." Published at HTTP://appliedclinicaltrialsonline.com on: March 17, 2016
- QUOTE: For several years, increasing numbers of life sciences organizations have implemented a Clinical Trial Management System (CTMS) that can provide insights gleaned from the system’s data to gain early and increased visibility into problems, progress and possibilities. Many organizations have a constant need to expand CTMS capabilities, integrate clinical operations data across multiple systems, and update clinical trial processes – all in order to adapt to changing regulatory requirements and clinical trial practices. ...
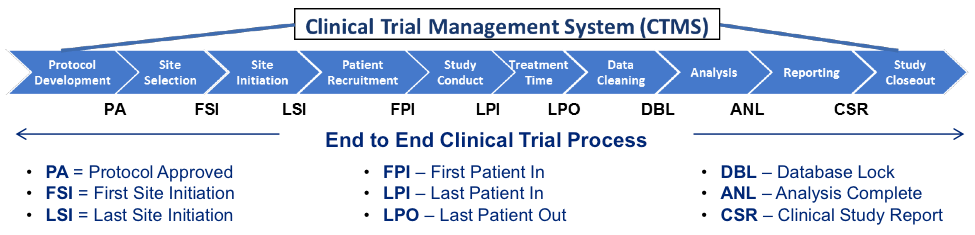
- A life sciences organization exploring the selection of a CTMS should anticipate the solution to provide the following core capabilities:
- Clinical program/project management - Enables oversight of related clinical trials per therapeutic area based on a set of specific clinical project activities (i.e., tracking actual vs. target). Includes the ability to track progress at specific trial and program levels.
- Trial and site planning - Facilitates investigator and site identification and recruitment, including key trial milestone tracking such as target site/enrollment metrics for each study country.
- Site and subject management - Provides tracking ability for site monitoring, subject enrollment relative to plan, and Case Report Form (CRF)/ Electronic Case Report Form (eCRF) completion status. Includes management of site visits/trip reports.
- Study management - Tracks key information such as CRF collection, Clinical Research Associate (CRA) monitoring frequency, protocol visit frequency, and adherence to protocol regiments. Includes support for study documentation and tracking tasks.
- Investigator management - Allows the trial sponsor to manage relationships with investigators, based on visibility into the status study data and related status with CROs and/or investigator sites. Also tracks approvals by Institutional Review Boards (IRB) and Independent Ethics Committees IEC(s).
- Study financials and investigator grants and payment management - Supports financial management including tracking study costs, reimbursing investigators, and paying claims related to study activities. Includes grant payment management and management of financial disclosure.
- Clinical supply management including supply tracking - Manages clinical supplies.
- Clinical trial performance and reporting - Provides reporting/dashboards to communicate trial performance against targets, as well as other operational reports.
- In addition, many current CTMS vendors are offering solutions with advanced capabilities for managing clinical trials that are beyond the scope of traditional CTMS solutions. Among these are:
- Protocol and study documentation management - Enables review/management and authoring of essential study documents. Also includes approval of clinical trial documentation and tracking its status throughout its lifecycle.
- CRF and eCRF development - Provides the ability to view and track the creation of CRF/eCRF forms including their status.
- Tracking electronic data capture (EDC) components and summary details - Tracks the status of data collected in these forms which, in turn, informs the tracking of key clinical trial milestones (e.g., Last Patient Visit (LPV).
- Financial system integration - Integrates SAP financial systems with pre-existing SAP workflows/business rules for payments, including invoicing approvals.
- Clinical supply management: storage and shipment - Extends clinical supply management to include oversight and management of clinical supply logistics for components such as inventory locations, lots, and shipment details.
- Clinical data archiving/warehousing and management - Provides enhanced data management and archiving for future reference
- Data analysis and query resolution - Supports the evaluation of trial-generated clinical data and resolution of any discrepancies or inconsistencies found in clinical data.
- Clinical learning and training - Integrates learning management to track and manage internal and external staff learning requirements relative to the clinical trial process.
- QUOTE: For several years, increasing numbers of life sciences organizations have implemented a Clinical Trial Management System (CTMS) that can provide insights gleaned from the system’s data to gain early and increased visibility into problems, progress and possibilities. Many organizations have a constant need to expand CTMS capabilities, integrate clinical operations data across multiple systems, and update clinical trial processes – all in order to adapt to changing regulatory requirements and clinical trial practices. ...