Clinical Trial Case Report Form (CRF)
A Clinical Trial Case Report Form (CRF) is a clinical record-keeping document that is designed to systematically collect a clinical study participant's clinical data.
- Context:
- It can (typically) be a person-related form that can be a printed, optical, or electronic document that contains all information required from a clinical study protocol for a single clinical trial participant.
- It can range from being a Paper CRF to being an eCRF/Electronic Case Report Form.
- It can be associated with a CRF Design Task, CRF Completion Manual, Data Management Plan, CRF Tracking.
- It can range from being a Clinical Trial CRF to being an Observational Clinical Study CRF.
- It can be used to facilitate Data Collection in a clinical trial.
- It can be designed to capture data in compliance with regulatory standards such as Good Clinical Practice.
- …
- Example(s):
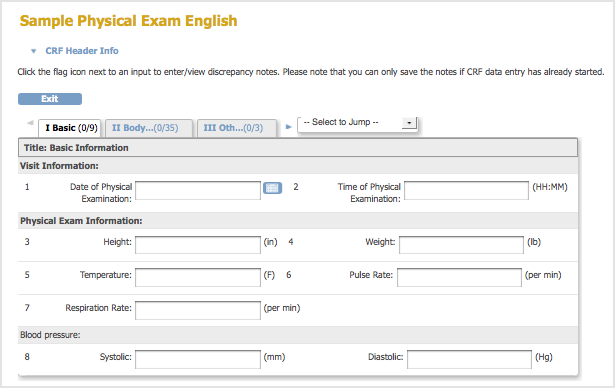
- A Sample Defined CRF when Viewed in the Web Interface:
- An FDA Form 1572, a CRF used in US clinical trials.
- A WHO Trial Registration Data Set, a standard set of CRF items used in international clinical research.
- …
- A Sample Defined CRF when Viewed in the Web Interface:
- Counter-Example(s):
- See: Clinical Study Protocol, Case Report, Clinical Outcome Assessment (COA), Clinical Research Casebook, Clinical Data Management, Data Validation.
References
2022
- https://medical-dictionary.thefreedictionary.com/case+report+form
- QUOTE:
- A printed, optical or electronic document designed to record all of the protocol-required information to be reported to the sponsor for each subject/patient in a clinical trial.
- A record of clinical study observations and other information that must be completed for each subject in a clinical trial, per study protocol mandate. CRF can refer to either a CRF page (which contains one or more data items linked together for collection and display) or a casebook (which includes all CRF pages on which a set of clinical study observations and other information can be or have been collected, or the information collected by completion of such CRF pages for a subject/patient in a clinical study). ...
- QUOTE:
2021a
- (Wikipedia, 2021) ⇒ https://en.wikipedia.org/wiki/Case_report_form Retrieved:2021-11-21.
- A case report form (or CRF) is a paper or electronic questionnaire specifically used in clinical trial research. The case report form is the tool used by the sponsor of the clinical trial to collect data from each participating patient. All data on each patient participating in a clinical trial are held and/or documented in the CRF, including adverse events.
The sponsor of the clinical trial develops the CRF to collect the specific data they need in order to test their hypotheses or answer their research questions. The size of a CRF can range from a handwritten one-time 'snapshot' of a patient's physical condition to hundreds of pages of electronically captured data obtained over a period of weeks or months. (It can also include required check-up visits months after the patient's treatment has stopped.)
The sponsor is responsible for designing a CRF that accurately represents the protocol of the clinical trial, as well as managing its production, monitoring the data collection and auditing the content of the filled-in CRFs.
Case report forms contain data obtained during the patient's participation in the clinical trial. Before being sent to the sponsor, this data is usually de-identified (not traceable to the patient) by removing the patient's name, medical record number, etc., and giving the patient a unique study number. The supervising Institutional Review Board (IRB) oversees the release of any personally identifiable data to the sponsor.
From the sponsor's point of view, the main logistic goal of a clinical trial is to obtain accurate CRFs. However, because of human and machine error, the data entered in CRFs is rarely completely accurate or entirely readable. To combat these errors monitors are usually hired by the sponsor to audit the CRF to make sure the CRF contains the correct data.
When the study administrators or automated mechanisms process the CRFs that were sent to the sponsor by local researchers, they make a note of queries. Queries are non-sensible or questionable data that must be explained. Examples of data that would lead to a query: a male patient being on female birth control medication or having had an abortion, or a 15-year-old participant having had hip replacement surgery. Each query has to be resolved by the individual attention of a member of each local research team, as well as an individual in the study administration. To ensure quality control, these queries are usually addressed and resolved before the CRF data is included by the sponsor in the final clinical study report. Depending on variables relating to the nature of the study, (e.g., the health of the study population), the effectiveness of the study administrators in resolving these queries can significantly impact the cost of studies.
- A case report form (or CRF) is a paper or electronic questionnaire specifically used in clinical trial research. The case report form is the tool used by the sponsor of the clinical trial to collect data from each participating patient. All data on each patient participating in a clinical trial are held and/or documented in the CRF, including adverse events.
2021b
- (Wikipedia) ⇒ https://en.wikipedia.org/wiki/Glossary_of_clinical_research#C Retrieved:2021-11-21.
- QUOTE: Case report form: A printed, optical, or electronic document designed to record all of the protocol-required information to be reported to the sponsor on each trial subject. (ICH E6)
2021c
- (NIA/NIH, 2021) ⇒ https://www.nia.nih.gov/research/dgcg/nia-glossary-clinical-research-terms Retrieved:2021-11-21.
- QUOTE: Case Report Form (CRF) – A printed, optical, or electronic (eCRF) document designed to capture all protocol-required information for a study.
2019
- (CD BioSciences, 2021) ⇒ ttps://www.cd-biosciences.com/crf-design/
- QUOTE: The case report form (CRF) is a paper or electronic questionnaire specifically used in clinical trial research, and is a record of the clinical trial data. The relevant data recorded in the clinical trial of the subject should be recorded in case report form that is strictly designed according to the requirements of the test protocol in advance. The CRF is like a loopback system of clinical study, which delivers the solution to each part in the most appropriate way, and collects, stores and transmits the scientific data.
The information and data in the CRF are from and consistent with the original document. All information required by the clinical trial should be filled in the CRF. The investigator should ensure that any observations and findings in the trial are recorded in the medical records in a timely, accurate, standardized and authentic manner and correctly entered into the CRF. CRF design is crucial in clinical trials as it will aid in assessing the safety and efficacy of the medicinal product accurately.

- QUOTE: The case report form (CRF) is a paper or electronic questionnaire specifically used in clinical trial research, and is a record of the clinical trial data. The relevant data recorded in the clinical trial of the subject should be recorded in case report form that is strictly designed according to the requirements of the test protocol in advance. The CRF is like a loopback system of clinical study, which delivers the solution to each part in the most appropriate way, and collects, stores and transmits the scientific data.
2014
- (Bellary et al., 2014) ⇒ Shantala Bellary, Binny Krishnankutty, and M. S. Latha (2014). "Basics of case report form designing in clinical research". In: Perspectives in Clinical Research, 5(4).
- QUOTE: Case report form (CRF) is a specialized document in clinical research. It should be study protocol driven, robust in content and have material to collect the study specific data. Though paper CRFs are still used largely, use of electronic CRFs (eCRFS) are gaining popularity due to the advantages they offer such as improved data quality, online discrepancy management and faster database lock etc. Main objectives behind CRF development are preserving and maintaining quality and integrity of data. CRF design should be standardized to address the needs of all users such as investigator, site coordinator, study monitor, data entry personnel, medical coder and statistician. Data should be organized in a format that facilitates and simplifies data analysis. Collection of large amount of data will result in wasted resources in collecting and processing it and in many circumstances, will not be utilized for analysis. Apart from that, standard guidelines should be followed while designing the CRF. CRF completion manual should be provided to the site personnel to promote accurate data entry by them. These measures will result in reduced query generations and improved data integrity. It is recommended to establish and maintain a library of templates of standard CRF modules as they are time saving and cost-effective. This article is an attempt to describe the methods of CRF designing in clinical research and discusses the challenges encountered in this process.