Clinical Outcome Assessment (COA) Instrument
(Redirected from Clinical outcome assessment (COA) tool)
Jump to navigation
Jump to search
A Clinical Outcome Assessment (COA) Instrument is a standardized formal perception-based clinical assessment instrument that quantifies a clinical study participant’s health outcomes.
- AKA: Health Outcome Assessment.
- Context:
- output: COA Score that produces COA values)
- It can (typically) be composed of a COA Questionnaire and a COA Scoring Method (input to a COA scoring task).
- It can (typically) be referenced in a Clinical Study Protocol.
- It can be instantiated into a COA-Study instance.
- It can range from being a Paper-based COA to being a Electronic COA (eCOA).
- It can range from being a Patient-Reported Outcome (PRO), a Clinician Reported Outcome (ClinRO), or an Observer Reported Outcome (ObsRO), based on who recorded the perception-based information (participant, clinician, or observer).
- It can range from being a Performance Outcome (PerfO) to being a Non-Performance Outcome Assessment, based on whether some activity must be performed.
- It can range from being a Experimentally Validated COA to being a Non-Validated COA.
- It can range from being a Symptom-focused COA, Function-focused COA, Quality-of-Life-focused COA, or Health-focused COA, based on whether it is intended to measure a participant's health symptoms, human function, human QoL , and other health outcomes.
- It can be used to evaluate the effectiveness of Drugs, Devices, and Procedures.
- It can be used in real-world clinical practice to monitor Patient Outcomes.
- …
- Example(s):
- an FDA Qualified Clinical Outcome Assessment (COA) such as:
- a Patient-Reported Outcome (PRO) such as: a Patient Health Questionnaire (PHQ-9),
- a Clinician-Reported Outcome (ClinRO), such as: a Clinical Global Impressions Scale (CGI), or a Unified Parkinson's Disease Rating Scale (UPDRS), or a Columbia Suicide Severity Rating Scale (C-SSRS).
- an Observer Reported Outcome (ObsRO), such as: a Behavior Assessment System for Children (BASC), or a Conners Comprehensive Behavior Rating Scales (CBRS),
- a Performance Outcome (PerfO), such as: a 6-Minute Walk Test (6MWT), or a Timed Up and Go Test (TUG),
- …
- Counter-Example(s):
- a Clinical Patient Diary, such as a food diary.
- a Clinical Procedure Test.
- a Clinical Procedure.
- a Adverse Event Measure (of adverse events).
- a Therapeutic Treatment Event.
- See: Case Report Form (CRF), Clinical Research Instrument, Clinical Trial Validated Instrument, Structured Health-Related Interview Questionnaire, Health-Related Unobservable Concept, Patient Intervention Questionnaire.
References
2023
Clinical Outcome Assessment (COA) is a type of measurement used in clinical trials and healthcare research to assess the impact of medical interventions on patients' health outcomes.
- It can be used to measure patients' Symptoms, Function, Quality of Life, and other Health Outcomes.
- It can be reported by Patients, Clinicians, or other Observers.
- It can be used to evaluate the effectiveness of Drugs, Devices, and Procedures.
- It can be used in real-world clinical practice to monitor patient outcomes.
2021a
- (FDA, 2021) ⇒ https://www.fda.gov/about-fda/clinical-outcome-assessment-coa-frequently-asked-questions#COADefinition
- QUOTE: A clinical outcome assessment is a measure that describes or reflects how a patient feels, functions, or survives. Types of COAs include:
2021b
- (FDA, 2021) ⇒ https://www.fda.gov/drugs/clinical-outcome-assessment-coa-qualification-program/qualified-clinical-outcome-assessments-coa Retrieved:2021-12-30.
- QUOTE: The table below lists qualified Clinical Outcome Assessments (COA). The tables include legacy projects (those submitted prior to passage of the 21st Century Cures Act), as well as those submitted as part of the newer section 507 process (referring to section 507 of the Federal Food, Drug and Cosmetic Act (FD&C Act)), which was created by Section 3011 of the 21st Century Cures Act). The tables are updated on a biannual basis and provide information on the COA qualification project, qualification statement, and supporting information. Additionally, the table includes documents from requestors that were received after the passage of the 21st Century Cures Act.
2021c
- (Physio-pedia, 2021) ⇒ https://www.physio-pedia.com/Clinical_Outcome_Assessment
- QUOTE:
- An outcome assessment, the patient assessment used in an endpoint, is the measuring instrument that provides a rating or score (categorical or continuous) that is intended to represent some aspect of the patient’s health status.
- Outcome assessments are used to define efficacy endpoints when developing a therapy for a disease or condition.
- Most efficacy endpoints are based on specified clinical assessments of patients.
- When clinical assessments are used as clinical trial outcomes, they are called clinical outcome assessments (COAs).
- QUOTE:
2020
- (Medable, 2020) ⇒ https://blog.medable.com/author/dr-ingrid-oakley-girvan
- QUOTE: ... In patient-centric clinical trials, COAs are essential to understand the impacts of a drug on certain endpoints, for example, whether it is improving or diminishing quality of life and everyday activities that matter to patients. The FDA, signaling the importance of COAs and the need to develop additional ones, has even created specific guidance and pathways for their development and validation. ...
...
- QUOTE: ... In patient-centric clinical trials, COAs are essential to understand the impacts of a drug on certain endpoints, for example, whether it is improving or diminishing quality of life and everyday activities that matter to patients. The FDA, signaling the importance of COAs and the need to develop additional ones, has even created specific guidance and pathways for their development and validation. ...
2019
- (NHI, 2019) ⇒ https://nationalhealthcouncil.org/blog/blog-fdas-roadmap-patient-focused-outcome-measurement-clinical-trials/
- QUOTE: ... Patient groups can help advance patient-centered drug development by the having information needed in column one at the ready. That can save considerable time and effort to speed the process and ensure the patient voice is accurately captured. ...
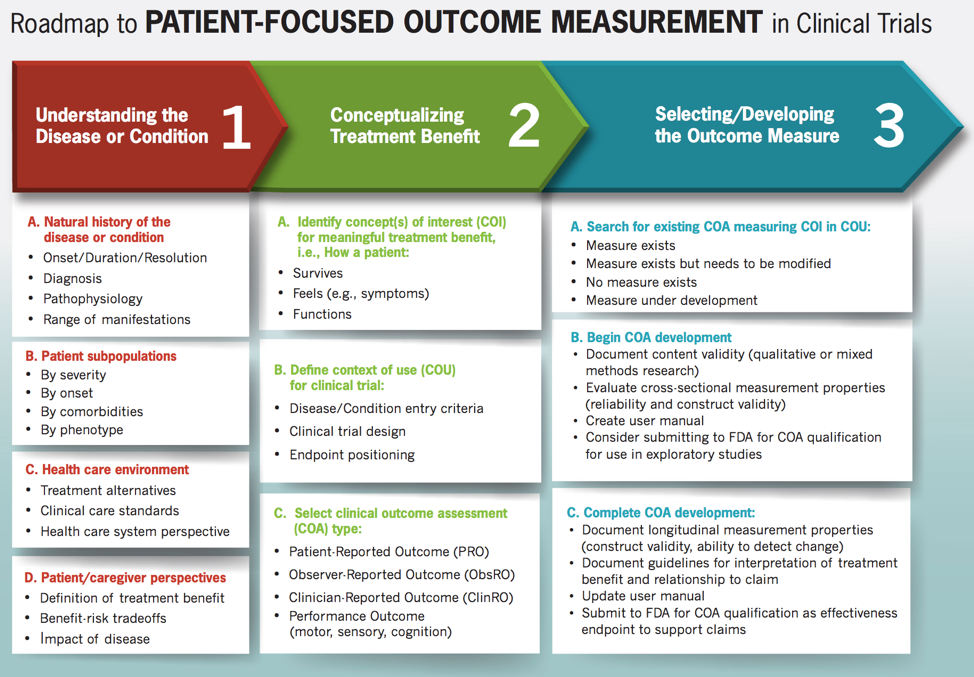
- QUOTE: ... Patient groups can help advance patient-centered drug development by the having information needed in column one at the ready. That can save considerable time and effort to speed the process and ensure the patient voice is accurately captured. ...
2016
- (Blakeley et al., 2016) ⇒ Jaishri O. Blakeley, Stephen Joel Coons, John R. Corboy, Nancy Kline Leidy, Tito R. Mendoza, and Jeffrey S. Wefel (2016). "Clinical Outcome Assessment in Malignant Glioma Trials: Measuring Signs, Symptoms, and Functional limitations". In: Neuro-Oncology, Volume 18, Issue suppl_2. DOI:10.1093/neuonc/nov291.
- QUOTE: Clinical outcome assessment (COA) tools, including measures of patient-reported outcome, performance outcome, clinician-reported outcome, and observer-reported outcome, allow patient-focused assessments to complement traditional efficacy measures such as overall survival and radiographic endpoints. This review examines the properties of various COA measures used in malignant glioma clinical trials to date and cross references their content to the priority signs, symptoms, and functional limitations defined through a community survey conducted by the National Brain Tumor Society (...)
There are several types of COAs, including PROs, performance outcomes (PerfOs), clinician-reported outcomes (ClinRO), and observer-reported outcomes (ObsROs) (Table 1 and Fig. 1).
- QUOTE: Clinical outcome assessment (COA) tools, including measures of patient-reported outcome, performance outcome, clinician-reported outcome, and observer-reported outcome, allow patient-focused assessments to complement traditional efficacy measures such as overall survival and radiographic endpoints. This review examines the properties of various COA measures used in malignant glioma clinical trials to date and cross references their content to the priority signs, symptoms, and functional limitations defined through a community survey conducted by the National Brain Tumor Society (...)