Empirical Formula
An Empirical Formula is a molecular formula that consists of the simplest positive integer ratio of atoms present in a chemical compound.
- AKA: Molecular Empirical Formula, Chemical Empirical Formula.
- …
- Example(s):
- C3H7, for Hexane.
- An Empirical Formula Mass.
- Counter-Example(s):
- C6H14, Molecular Formula for Hexane.
- Chemical Compound Percent Composition.
- See: Molecular Formula, Empirical, Formula, Elemental Analysis, Chemical Compound, Integer, Ratio, Atom, Sulfur Monoxide, Disulfur Dioxide, Sulfur, Oxygen, Chemical Formula, Ionic Compound.
References
2017a
- (Wikipedia, 2017) ⇒ https://en.wikipedia.org/wiki/Empirical_formula Retrieved:2017-7-2.
- In chemistry, the empirical formula of a chemical compound is the simplest positive integer ratio of atoms present in a compound. A simple example of this concept is that the empirical formula of sulfur monoxide, or SO, would simply be SO, as is the empirical formula of disulfur dioxide, S2O2. This means that sulfur monoxide and disulfur dioxide, both compounds of sulfur and oxygen, will have the same empirical formula. However, their chemical formulas, which express the number of atoms in each molecule of a chemical compound, will not be the same.
An empirical formula makes no mention of the arrangement or number of atoms. It is standard for many ionic compounds, like calcium chloride (CaCl2), and for macromolecules, such as silicon dioxide (SiO2).
The molecular formula, on the other hand, shows the number of each type of atom in a molecule. The structural formula shows the arrangement of the molecule. It is also possible for different types of compounds to have equal empirical formulas.
Samples are analyzed in specific elemental analysis tests to determine what percent of a particular element the sample is composed of.
- In chemistry, the empirical formula of a chemical compound is the simplest positive integer ratio of atoms present in a compound. A simple example of this concept is that the empirical formula of sulfur monoxide, or SO, would simply be SO, as is the empirical formula of disulfur dioxide, S2O2. This means that sulfur monoxide and disulfur dioxide, both compounds of sulfur and oxygen, will have the same empirical formula. However, their chemical formulas, which express the number of atoms in each molecule of a chemical compound, will not be the same.
2017b
- (De Leon, 2017) ⇒ http://www.iun.edu/~cpanhd/C101webnotes/quantchem/empiricalform.html Retrieved:2017-7-2.
- QUOTE: The empirical formula of a molecular compound is the simplest possible formula. The empirical formula contains the correct ratio of atoms in the compound but not necessarily the correct number of atoms. We discussed this earlier. The empirical formula can be obtained from an experimental analysis of the percent composition of the compound. If the mass percent composition of the compound is experimentally determined, we can then use the mole concept to get the empirical formula. For example, consider carboxylic acid. Experimentally it is determined that carboxylic acid contains C(41.4%), O(55.2%)and O. To use this information to determine the empirical formula we first imagine 100 g of carboxylic acid. This 100 g number is convenient because the percentages will then reflect the mass of each element in 100 g of the compound. Therefore we have, in 100 g of carboxylic acid, 41.4 g C, 55.12 g O and 3.48 g of H. (...)
2017c
- (OpenSTAX, 2017) ⇒ OpenSTAX: Determining Empirical and Molecular Formulas Retrieved:2017-07-08
- QUOTE: As previously mentioned, the most common approach to determining a compound’s chemical formula is to first measure the masses of its constituent elements. However, we must keep in mind that chemical formulas represent the relative numbers, not masses, of atoms in the substance. Therefore, any experimentally derived data involving mass must be used to derive the corresponding numbers of atoms in the compound. To accomplish this, we can use molar masses to convert the mass of each element to a number of moles. We then consider the moles of each element relative to each other, converting these numbers into a whole-number ratio that can be used to derive the empirical formula of the substance. Consider a sample of compound determined to contain 1.71g C and 0.287g H. The corresponding numbers of atoms (in moles) are:
- [math]\displaystyle{ 1.17\;g\;C\times \frac{1\;mol\;C}{12.01g\;C}=0.142\;mol\;C \quad }[/math](3.2.10)
- [math]\displaystyle{ 0.287\;g\;H\times\frac{1\;mol\;H}{1.008\;g\;H}=0.284\;mol\;H\quad }[/math](3.2.11)
- QUOTE: As previously mentioned, the most common approach to determining a compound’s chemical formula is to first measure the masses of its constituent elements. However, we must keep in mind that chemical formulas represent the relative numbers, not masses, of atoms in the substance. Therefore, any experimentally derived data involving mass must be used to derive the corresponding numbers of atoms in the compound. To accomplish this, we can use molar masses to convert the mass of each element to a number of moles. We then consider the moles of each element relative to each other, converting these numbers into a whole-number ratio that can be used to derive the empirical formula of the substance. Consider a sample of compound determined to contain 1.71g C and 0.287g H. The corresponding numbers of atoms (in moles) are:
- Thus, we can accurately represent this compound with the formula [math]\displaystyle{ C_{0.142}H_{0.248} }[/math]. Of course, per accepted convention, formulas contain whole-number subscripts, which can be achieved by dividing each subscript by the smaller subscript:
- [math]\displaystyle{ C_{0.142/0.142}H_{0.248/0.142} }[/math] or [math]\displaystyle{ CH_2\quad }[/math](3.2.12)
- (Recall that subscripts of “1” are not written, but rather assumed if no other number is present.)
- The empirical formula for this compound is thus [math]\displaystyle{ CH_2 }[/math]. This may or not be the compound’s molecular formula as well; however, we would need additional information to make that determination (as discussed later in this section).
- Consider as another example: a sample of compound determined to contain [math]\displaystyle{ 5.31\;g\;Cl }[/math] and [math]\displaystyle{ 8.40\;g\;O }[/math]. Following the same approach yields a tentative empirical formula of:
- * [math]\displaystyle{ Cl_{0.150}O_{0.525}=Cl_{0.150/0.150}O_{0.525/0.150}=ClO_{3.5} }[/math](3.2.13)
- In this case, dividing by the smallest subscript still leaves us with a decimal subscript in the empirical formula. To convert this into a whole number, we must multiply each of the subscripts by two, retaining the same atom ratio and yielding [math]\displaystyle{ Cl_2O_7 }[/math] as the final empirical formula.
- In summary, empirical formulas are derived from experimentally measured element masses by:
- Deriving the number of moles of each element from its mass
- Dividing each element’s molar amount by the smallest molar amount to yield subscripts for a tentative empirical formula
- Multiplying all coefficients by an integer, if necessary, to ensure that the smallest whole-number ratio of subscripts is obtained
- Figure 3.2.13.2.1 outlines this procedure in flow chart fashion for a substance containing elements A and X.
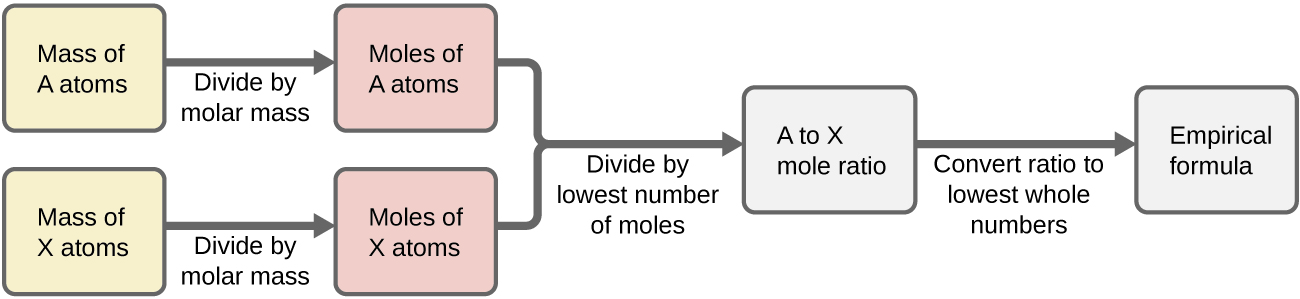
- (...) Finally, with regard to deriving empirical formulas, consider instances in which a compound’s percent composition is available rather than the absolute masses of the compound’s constituent elements. In such cases, the percent composition can be used to calculate the masses of elements present in any convenient mass of compound; these masses can then be used to derive the empirical formula in the usual fashion.
- Thus, we can accurately represent this compound with the formula [math]\displaystyle{ C_{0.142}H_{0.248} }[/math]. Of course, per accepted convention, formulas contain whole-number subscripts, which can be achieved by dividing each subscript by the smaller subscript: