Noninferiority Clinical Trial
A Noninferiority Clinical Trial is a Clinical Trial which primary objective is to show that the response to the investigational product (or new treatment) is not clinically inferior to a comparative agent.
- Example(s):
- NCT00465816: Non-inferiority of Nimenrix (Meningococcal vaccine 134612),
- NCT03999944: Non-Inferiority of the FRESCA Airbox Positive Airway Pressure System (Sleep Apnea),
- NCT02680834: Non-Inferiority of a Dual Action Pneumatic Compression Device and Multi-Layer Bandaging (Venous Leg Ulcer),
- NCT02233803:Non-inferiority of NEUMOTEROL-400 and SYMBICORT-Forte (Asthma),
- …
- Counter-Example(s):
- See: Randomized Clinical Trial, Clinical Trial Participant, Clinical Trial Design, Phase 1 Clinical Trial, Phase 2 Clinical Trial, Phase 3 Clinical Trial, Placebo-Controlled Clinical Trial, Drug Development Clinical Trial, Double-Blinded Clinical Trial.
References
2021
- (Wikipedia, 2021) ⇒ https://en.wikipedia.org/wiki/Glossary_of_clinical_research#N Retrieved:2022-01-10.
- QUOTE: Non-inferiority trial: A trial with the primary objective of showing that the response to the investigational product is not clinically inferior to a comparative agent (active or placebo control). (ICH E9)
2017
- (Wang et al., 2017) ⇒ Bokai Wang, Hongyue Wang, Xin M. Tu, and Changyong Feng (2017) "Comparisons of Superiority, Non-inferiority, and Equivalence Trials". In: Shanhai Archives of Psychiatry, 29(6). DOI:10.11919/j.issn.1002-0829.217163.
- QUOTE: A non-inferiority trial is to show that treatment A is not worse than the treatment B. Although these kinds of trials are not used to establish better treatment efficacy, the new method may have advantages over current methods in other aspects. For example, the new intervention may be less costly, less invasive, and have less side effects.
The hypotheses of non-inferiority clinical trials are
- QUOTE: A non-inferiority trial is to show that treatment A is not worse than the treatment B. Although these kinds of trials are not used to establish better treatment efficacy, the new method may have advantages over current methods in other aspects. For example, the new intervention may be less costly, less invasive, and have less side effects.
| $H_0:\mu_1-\mu_0\leq-\delta$ vs. $H_1:\mu_1-\mu_0>-\delta$ | (5) |
- where $\delta \geq 0$ and is also called the margin of clinical significance which is usually small.
The non-inferiority of the treatment to the control can be easily understood form the alternative hypothesis. If the mean difference between the treatment and control group is greater than $\delta$, then the treatment is non-inferior to the control. Unlike the superiority trial, we don’t need the treatment to be better than the control.
- where $\delta \geq 0$ and is also called the margin of clinical significance which is usually small.
2011
- (Walker & Nowacki, 2011) ⇒ Esteban Walker, and Amy S. Nowacki (2011) "Understanding Equivalence and Noninferiority Testing". In: Springer - Journal of General Internal Medicine, 26(2). DOI:10.1007/s11606-010-1513-8.
- QUOTE: The determination of the equivalence margin, $\delta$, is the most critical step in equivalence/noninferiority testing. A small value of $\delta$ determines a narrower equivalence region, and makes it more difficult to establish equivalence/noninferiority. The equivalence margin not only determines the result of the test, but also gives scientific credibility to a study. The value and impact of a study depend on how well the equivalence margin can be justified in terms of relevant evidence and sound clinical considerations. Frequently, regulatory issues also have to be considered.[1]
An equivalence/noninferiority study should be designed to minimize the possibility that a new therapy that is found to be equivalent/noninferior to the current therapy can be nonsuperior to a placebo. One way to minimize this possibility is to choose a value of the equivalence margin based on the margin of superiority of the current therapy against the placebo. This margin of superiority can be estimated from previous studies. In noninferiority testing, a common practice is to set the value of $\delta$ to a fraction, $f$, of the lower limit of a confidence interval of the difference between the current therapy and the placebo obtained from a meta-analysis. The smaller the value of $f$, the more difficult the establishment of equivalence/noninferiority of the new therapy.
- QUOTE: The determination of the equivalence margin, $\delta$, is the most critical step in equivalence/noninferiority testing. A small value of $\delta$ determines a narrower equivalence region, and makes it more difficult to establish equivalence/noninferiority. The equivalence margin not only determines the result of the test, but also gives scientific credibility to a study. The value and impact of a study depend on how well the equivalence margin can be justified in terms of relevant evidence and sound clinical considerations. Frequently, regulatory issues also have to be considered.[1]
- ↑ Garret AD. Therapeutic equivalence: fallacies and falsification. Statist Med. 2003;22:741–762. doi: 10.1002/sim.1360.
2009
- (Greene et al., 2009) ⇒ Carolyn J. Greene, Leslie A. Morland, Valerie L. Durkalski, and B. Christopher Frueh (2009). "Noninferiority and Equivalence Designs: Issues and Implications for Mental Health Research". In: Journal of Traumatic Stress - ISTSS Willey, 21(5). DOI:10.1002/jts.20367.
- QUOTE: Noninferiority and equivalence designs are becoming increasingly common and relevant in randomized controlled trials (RCTs) in the fields of medicine and mental health. Noninferiority designs are a one-sided test used to determine if a novel intervention is no worse than a standard intervention. Equivalence designs, a two-sided test, pose a similar question, but also allow for the possibility that the novel intervention is no better than the standard one. Issues related to the comparability between novel interventions and better established ones are of great importance to improving health service delivery across a wide range of settings and contexts. However, noninferiority and equivalence designs are often poorly understood, improperly applied, and subsequently misinterpreted. These designs differ from traditional “superiority” trials in significant conceptual and statistical ways and pose a unique set of challenges to investigators and consumers of research(...)
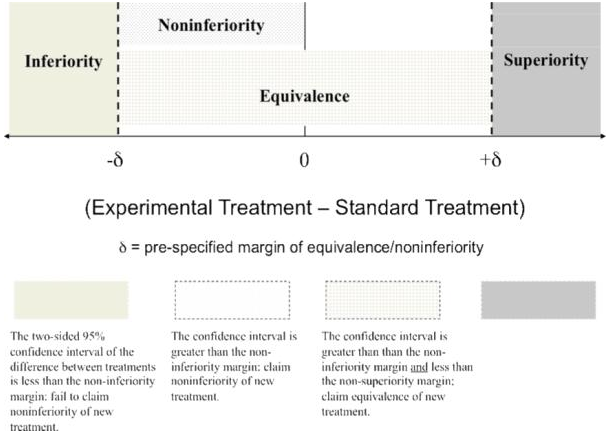
Figure 1 provides an illustration of this zone. Equivalency designs are rarely used in therapeutic trials evaluating effectiveness since the study objective often is to show that a new treatment is not inferior to a standard, which corresponds to a noninferiority design, as opposed to showing that the novel treatment is neither inferior nor superior to the standard, which corresponds to the equivalency design. Because noninferiority trials are much more common in mental health research, this article focuses on that design.
- QUOTE: Noninferiority and equivalence designs are becoming increasingly common and relevant in randomized controlled trials (RCTs) in the fields of medicine and mental health. Noninferiority designs are a one-sided test used to determine if a novel intervention is no worse than a standard intervention. Equivalence designs, a two-sided test, pose a similar question, but also allow for the possibility that the novel intervention is no better than the standard one. Issues related to the comparability between novel interventions and better established ones are of great importance to improving health service delivery across a wide range of settings and contexts. However, noninferiority and equivalence designs are often poorly understood, improperly applied, and subsequently misinterpreted. These designs differ from traditional “superiority” trials in significant conceptual and statistical ways and pose a unique set of challenges to investigators and consumers of research(...)
|