Superiority Clinical Trial
A Superiority Clinical Trial is a Clinical Trial which primary objective is to show that the response to the investigational product (or new treatment) is clinically superior to a comparative agent.
- Example(s):
- Counter-Example(s):
- See: Randomized Clinical Trial, Clinical Trial Participant, Clinical Trial Design, Phase 1 Clinical Trial, Phase 2 Clinical Trial, Phase 3 Clinical Trial, Placebo-Controlled Clinical Trial, Drug Development Clinical Trial, Double-Blinded Clinical Trial.
References
2021
- (Wikipedia, 2021) ⇒ https://en.wikipedia.org/wiki/Glossary_of_clinical_research#S Retrieved:2022-01-10.
- QUOTE: Superiority trial
- A trial with the primary objective of showing that the response to the investigational product is superior to a comparative agent (active or placebo control). (ICH E9)
2017
- (Wang et al., 2017) ⇒ Bokai Wang, Hongyue Wang, Xin M. Tu, and Changyong Feng (2017) "Comparisons of Superiority, Non-inferiority, and Equivalence Trials". In: Shanhai Archives of Psychiatry, 29(6). DOI:10.11919/j.issn.1002-0829.217163.
- QUOTE: In a superiority trial, we want to show that the new treatment intervention (drug, psychotherapy) is superior to (better than) the control condition. For example, we want to know if a new drug can significantly increase CD4 counts for HIV patients or a novel psychosocial therapy will increase social activities for lonely old adults.
For many researchers, a challenging problem is how to specify the null and alternative hypotheses for the specific trial. A rule of thumb is to specify the null hypothesis opposite to what we expect for the outcome. For example, if we want to test if treatment A is better than treatment B, the null hypothesis is that A is not better than or same as B. We anticipate that the data from the trial will tell us otherwise and reject the null hypothesis in support of the anticipated superiority of treatment A. Based on this idea, the null and alternative hypotheses of a superiority trial are specified as
- QUOTE: In a superiority trial, we want to show that the new treatment intervention (drug, psychotherapy) is superior to (better than) the control condition. For example, we want to know if a new drug can significantly increase CD4 counts for HIV patients or a novel psychosocial therapy will increase social activities for lonely old adults.
|
$H_0:\mu_1-\mu_0\leq \delta$ vs. $H_1:\mu_1-\mu_0>\delta$ |
(1) |
- where $\delta \geq$(...)
The value $\delta$ in (1) is called margin of clinical significance.
- where $\delta \geq$(...)
2009
- (Greene et al., 2009) ⇒ Carolyn J. Greene, Leslie A. Morland, Valerie L. Durkalski, and B. Christopher Frueh (2009). "Noninferiority and Equivalence Designs: Issues and Implications for Mental Health Research". In: Journal of Traumatic Stress - ISTSS Willey, 21(5). DOI:10.1002/jts.20367.
- QUOTE: Figure 1 provides an illustration of this zone (...)
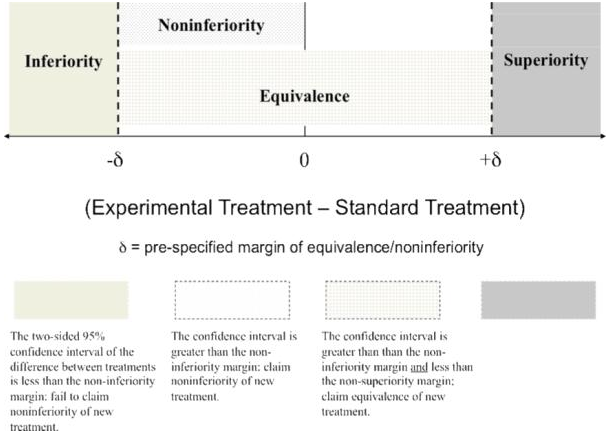
|
- Noninferiority designs differ from standard superiority trials in several fundamental ways. The null hypothesis of standard superiority trials is that there is no true difference between the interventions. In other words, unless strong evidence is found indicating the superiority of one intervention over the other, the default conclusion is that no treatment difference exists.