Decentralized Clinical Trial (DCT)
A Decentralized Clinical Trial (DCT) is a clinical trial that involves decentralized data collection (from patients).
- AKA: Direct-to-Participant Trial, Remote Decentralized Clinical Trial.
- Context:
- It can range from being a Small-Scale Decentralized Clinical Trial to being a Large-Scale Decentralized Clinical Trial.
- It can range from being a Randomized Decentralized Clinical Trial to being a Non-Randomized Decentralized Clinical Trial.
- It can range from being a Regional Decentralized Clinical Trial (such as an India Clinical Trial) to being a Global Clinical Trial.
- It can range from being a Government-lead Decentralized Clinical Trial to being an Organizational-led Decentralized Clinical Trial, such as a Pfizer-led DCT.
- It can (typically) be managed by a DCT System (such as a DCT SaaS).
- It can improve on CT Costs, CT Patient Recruitment, CT Patient Retention, and CT Data Quality.
- It can range from being a Fully Decentralized Clinical Trial to being Partially Decentralized Clinical Trial.
- It can be managed by a[ DCT CTMS.
- …
- Example(s):
- Counter-Example(s):
- See: Telemedicine, Digital Medicine, Telehealth, Contract Research Organization (CRO), Randomized Clinical Trial, Decentralized Research, Decentralized Computing, Decentralized Web, Decentralized System, Decentralization, Clinical Trials Transformation Initiative (CTTI), Association of Clinical Research Organizations (ACRO), COVID-19.
References
2021a
- (Babaian, 2021) ⇒ David Babaian (2021). "Defining Decentralized Clinical Trials and Understanding Their Nuances". In Stat+ News - ADVARRA.
- QUOTE: [[Decentralized clinical trials (DCT)]] are defined as studies executed through telemedicine and mobile local healthcare providers, using processes and technologies differing from the traditional clinical trial model.[1]
- ↑ Clinical Trial Transformation Initiative, CTTI Recommendations: Decentralized Clinical Trials 2 (2018), available at https://www.ctti-clinicaltrials.org/sites/www.ctti-clinicaltrials.org/files/dct_recommendations_final.pdf.
2021b
- (Bain, 2021) ⇒ Douglas Bain (2021)."Decentralized Clinical Trials: Opportunity or Challenge?". In: MassBio News.
- QUOTE: Clinical trials have typically leveraged standard of care methods. The only significant adjustment to methods used are that a patient might receive is the frequency of patient visits and the consent/screening process. With Decentralized Clinical Trial (DCT) methods applied, the trial might involve the patient using a sponsor-provided app on their own smartphone, using wearable sensors, and being consulted remotely using video conferencing by the investigator. Pre-pandemic, this sponsor-controlled switch in methods was difficult for many countries and regulators to actively support. As we emerge from a global pandemic experience, we are seeing an increasing realization that remote standard of care methods are not only possible, but in many cases preferable.
2021c
- (DTRA, 2021) ⇒ https://www.dtra.org Retrieved:2021-09-21.
- QUOTE: Decentralized Trials & Research Alliance Members represent the diverse ecosystem required to support the adoption of decentralized research methods.
Organizational Members include pharmaceutical and biotechnology companies, regulatory authorities, patient/advocacy organizations, clinical research organizations, technology companies, specialized service providers, investigator site networks, consulting organizations, and other stakeholders.
- QUOTE: Decentralized Trials & Research Alliance Members represent the diverse ecosystem required to support the adoption of decentralized research methods.
2021d
- (Everest Group) ⇒ https://www2.everestgrp.com/reportaction/EGR-2021-46-R-4459/Marketing
- QUOTE: Decentralized Clinical Trials (DCTs), in which clinical trial data is collected through sensors or remote monitoring devices carried by a patient without the need to visit a site, can deliver many benefits to pharmaceutical companies, including cost savings, better patient recruitment and retention, and improved data quality. ...
2021e
- (Henderson, 2021) ⇒ Zach Henderson (2021). “Decentralized Clinical Trials: Surviving & Thriving in the Clinical Research World of COVID-19" In: HIT Consultant.
- QUOTE: The quality - and efficiency-focused Clinical Trials Transformation Initiative (CTTI), has said that decentralized trials (DCTs) can follow a range of approaches, from fully decentralized trials to partially decentralized or hybrid trials, in which some aspects of a trial are conducted remotely while others are performed in person. CTTI notes that DCTs are trials consisting of any combination of certain core features, including no physical trial sites used in the trial, all visits performed via telemedicine or mobile/local HCPs, and/or data captured remotely through use of mobile technologies.
While DCTs offer several advantages over traditional in-person randomized, controlled trials (RCTs) and momentum for a shift towards DCTs have been growing gradually for years, the primary driver for moving toward the model prior to the pandemic has been its patient-centered focus. The DCT model is especially valuable for patient enrollment efforts, making clinical trials available to patients at non-investigative sites and even enabling participation from home. This broadens the number and diversity of eligible participants, as trials and their benefits are no longer limited to those living near the key urban centers where RCTs are traditionally based but are also open now to those living remotely or in rural areas.[1]
With the onset of COVID-19, researchers rapidly recognized that DCTs not only removed the burdens and geographic barriers of in-person trial participation but also offered the social distancing now required for the safety of their patients and trial staff. Suddenly, research companies saw the popularity of DCTs skyrocket[2], and a vast number of clinical trials have been revived due to the exponential adoption of the DCT model, without which many — if not most — of the impacted trials would still be at a standstill.
- QUOTE: The quality - and efficiency-focused Clinical Trials Transformation Initiative (CTTI), has said that decentralized trials (DCTs) can follow a range of approaches, from fully decentralized trials to partially decentralized or hybrid trials, in which some aspects of a trial are conducted remotely while others are performed in person. CTTI notes that DCTs are trials consisting of any combination of certain core features, including no physical trial sites used in the trial, all visits performed via telemedicine or mobile/local HCPs, and/or data captured remotely through use of mobile technologies.
- ↑ Clinical Trials Transformation Initiative. Decentralized Clinical Trials. Published September 2018. https://www.ctti-clinicaltrials.org/projects/decentralized-clinical-trials
- ↑ Ledford H. (2020). The coronavirus outbreak could make it quicker and easier to trial drugs. Nature, 582, 172. https://doi.org/10.1038/d41586-020-01524-0
2021f
- (Mittal et al., 2021) ⇒ Nitish Mittal, Chunky Satija, and Nisarg Shah (2021). “Decentralized Clinical Trial Products PEAK Matrix® Assessment 2021." Everest Group.
- QUOTE: ... Decentralized Clinical Trials (DCTs), in which clinical trial data is collected through sensors or remote monitoring devices carried by a patient without the need to visit a site, can deliver many benefits to pharmaceutical companies, including cost savings, better patient recruitment and retention, and improved data quality. Before the COVID-19 pandemic, although the technology and literature to support DCTs existed, there were only a few pilots being conducted as enterprises grappled with regulatory uncertainties, upfront capital investment in sensors and products, and limited functionalities to decentralize clinical trials. In recent times, DCTs have proved to be a saving grace to restart paused clinical trials. Additionally, recent technological advances, the proliferation of wearables, and FDA’s push to the industry to adopt DCTs following the COVID-19 situation have made the DCT landscape ripe for disruption. Numerous start-ups that address DCT requirements have recently emerged. The landscape has also experienced heavy fundraising and M&A activity. Through co-innovation, continuous product improvement, and market education, DCT vendors are focusing on increasing trust, speeding trial timelines, and delivering a smooth experience in running DCTs. ...
2021g
- (Van Norman, 2021) ⇒ Gail A. Van Norman. (2021). “Decentralized Clinical Trials The Future of Medical Product Development?â.” In: JACC: Basic to Translational Science - Elsevier Journal, 6(4). doi:10.1016/j.jacbts.2021.01.011.
- QUOTE: DCTs also termed “direct-to-participant trials" or "virtual" studies are characterized by less dependence on traditional research facilities or specialist intermediaries for data collection. DCTs leverage "virtual" tools, such as telemedicine, sensory-based technologies, wearable medical devices, home visits, patient-driven virtual health care interfaces, and direct delivery of study drugs and materials to patients' homes. In a fully decentralized clinical trial, subject recruitment, delivery and administration of study medication, and acquisition of trial outcomes data all proceed without involving in-person contact between the study team and the patient subject. Currently, clinical trials for drug approval often already include decentralized elements, and DCTs often incorporate traditional design with decentralization of the patient/subject interactions (Central Illustration).

|
2020a
- (Apostolaros et al., 2020) ⇒ Maria Apostolaros, David Babaian, Amy Corneli, Annemarie Forrest, Gerrit Hamre, Jan Hewett, Laura Podolsky, Vaishali Popat, and Penny Randall. (2020). “Legal, Regulatory, and Practical Issues to Consider When Adopting Decentralized Clinical Trials: Recommendations From the Clinical Trials Transformation Initiative.” In: Therapeutic Innovation & Regulatory Science Journal, 54(4).
- QUOTE: ... Traditional clinical trials are often expensive, inefficient, include selected populations, and can create significant participant burden via travel and other logistical demands. Using new technologies and methodologies to promote a decentralized approach has the potential to improve the efficiency of clinical trials. The Clinical Trials Transformation Initiative (CTTI) — a public–private partnership to improve clinical trials — launched a multi-stakeholder Decentralized Clinical Trials (DCTs) Project to provide recommendations on addressing the actual and perceived legal, regulatory, and practical challenges with DCT design and conduct in the United States. ...
CTTI’s recommendations cover protocol design, use of telemedicine and mobile healthcare providers, medical product supply chain, investigator delegation and oversight, and safety monitoring considerations. By implementing these recommendations, sponsors, contract research organizations, and others can help advance successful medical product development using mobile technologies and methodologies in DCTs. ...
- QUOTE: ... Traditional clinical trials are often expensive, inefficient, include selected populations, and can create significant participant burden via travel and other logistical demands. Using new technologies and methodologies to promote a decentralized approach has the potential to improve the efficiency of clinical trials. The Clinical Trials Transformation Initiative (CTTI) — a public–private partnership to improve clinical trials — launched a multi-stakeholder Decentralized Clinical Trials (DCTs) Project to provide recommendations on addressing the actual and perceived legal, regulatory, and practical challenges with DCT design and conduct in the United States. ...
2020b
- (Tan et al., 2020) ⇒ Aaron C. Tan, David M. Ashley, and Mustafa Khasraw. (2020). “Adapting to a Pandemic â Conducting Oncology Trials During the SARS-CoV-2 Pandemic.” In: American Association for Cancer Research Journal. ISBN:1557-3265 doi:10.1158/1078-0432.CCR-20-1364.
- QUOTE: ... Furthermore, the burgeoning costs associated with conducting large randomized trials shows no signs of slowing downCite error: Invalid
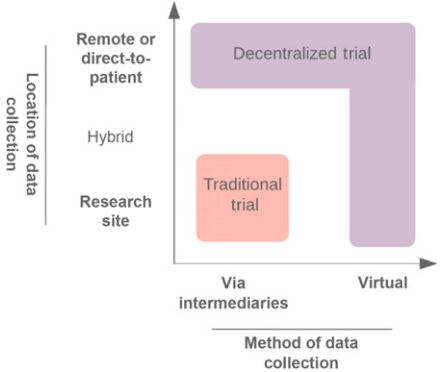
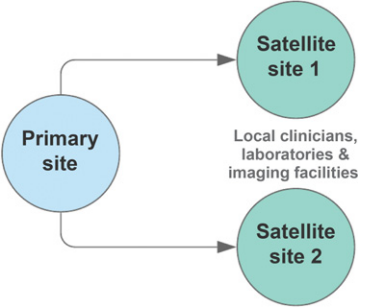
<ref>tag; invalid names, e.g. too many. A move toward a more decentralized clinical trial model (Fig. 1) or satellite sites (Fig. 2) could improve the adaptability of trials. Indeed, there have been ongoing efforts in this space[1]. Decentralized clinical trials represent a concept that data can be collected at remote locations, and the method of data collection may also be virtual[2]. This is in contrast to traditional clinical trials, where data must be collected at the designated research facility and via intermediaries such as CROs. Importantly, decentralized trials do not compromise on study design or statistical consideration. It refers predominantly to the locality and method of data collection. Accordingly, protocol-specified procedures compliant with regulatory requirements may still be conducted. The FDA's increasing focus on real-world evidence (RWE), to generate evidence and data outside the tools and methods of traditional trial settings, is recognition of the unharnessed potential to capture multiple data sources[3].
- QUOTE: ... Furthermore, the burgeoning costs associated with conducting large randomized trials shows no signs of slowing downCite error: Invalid
|
|
- ↑ Sabesan S, Zalcberg J. Telehealth models could be extended to conducting clinical trials-a teletrial approach. Eur J Cancer Care 2018;27:e12587.
- ↑ Coravos A, Goldsack JC, Karlin DR, Nebeker C, Perakslis E, Zimmerman N, et al. Digital medicine: a primer on measurement. Digit Biomark 2019;3: 31–71.
- ↑ Sherman RE, Anderson SA, Dal Pan GJ, Gray GW, Gross T, Hunter NL, et al. Real-world evidence - what is it and what can it tell us? N Engl J Med 2016;375: 2293–7.
- ↑ Khozin S, Pazdur R, Shah A. INFORMED: an incubator at the US FDA for driving innovations in data science and agile technology. Nat Rev Drug Discov 2018;17:529–30.