Software as a Medical Device (SaMD)
(Redirected from SaMD)
Jump to navigation
Jump to search
A Software as a Medical Device (SaMD) is a medical device that is medical software (it has been designed and developed to carry out medical functions without the need for computer hardware).
- Context:
- It can be comprised of software or applications intended for disease screening, medical diagnosing, and treatment.
- It can be used in combination and interfaced with other medical devices, including hardware medical devices, and other (SaMD or general purpose) software.
- It can be supported by a SaMD System (supported by a SaMD platform, such as Medable SaMD platform).
- It can be evaluated by a SaMD Evaluation Clinical Trial.
- It is required to follow SaMD Quality Management Principles.
- It is categorized according to a SaMD Definition Statement.
- It can range from being a SaMD Category I to being a SaMD Category IV.
- ...
- Example(s):
- Mobile SaMD App.
- Patient Data SaMD.
- Image Data SaMD.
- Treatment Data SaMD.
- Sleep Data SaMD.
- Breast Screening SaMD.
- a software-supported In-Vitro Diagnostic (IVD) Medical Device.
- ...
- …
- Counter-Example(s):
- See: Medable, Inc., Medical Wearable Device, Biosensor, Telemedicine, Decentralized Clinical Trial, Direct-to-Patient Digital Trials Platform, End-to-End Clinical Research Platform, SaaMD Design History File.
References
- https://infomeddnews.com/5-software-as-a-medical-device-examples/
- QUOTE: ... Here are five examples of SaMD to further explain how these devices assist medical professions in collecting the data they need to make better medical decisions to help patients recover from various types of health issues.
- Patient Data SaMD: Several SaMD fall under this heading and include such things as the software used to collect patient data in real-time measurements that can be displayed and monitored remotely by a medical professional. The data may include heart rate stats, blood pressure readings, and other similar pieces of vital statistics.
- Image Data SaMD: By analyzing and manipulating images and data collected from radiation-emitting imaging devices, this software can create 3D models. ...
- Treatment Data SaMD: Patient information is analyzed with this type of new technology software that is used as an algorithm. ...
- Sleep Data SaMD: Rather than using a sleep lab setting, this software analyzes the physiological signals collected when a patient is sleeping. ...
- Breast Screening SaMD: This software uses the identical digital mammogram that radiologists use and calculates breast density percentage with it. ...
- QUOTE: ... Here are five examples of SaMD to further explain how these devices assist medical professions in collecting the data they need to make better medical decisions to help patients recover from various types of health issues.
2019
- (Select Hub, 2019) ⇒ https://www.selecthub.com/medical-software/software-medical-device-samd/
- QUOTE: ... Software as a Medical Device, or SaMD, can be described as a class of medical software designed to carry out one or more medical functions without the need for actual hardware. This can comprise of software or applications intended to treat diagnose, cure, mitigate or prevent disease. SaMD is typically used with non-medical computing platforms connected to virtual networks or other general-use hardware.
 ...
...
- QUOTE: ... Software as a Medical Device, or SaMD, can be described as a class of medical software designed to carry out one or more medical functions without the need for actual hardware. This can comprise of software or applications intended to treat diagnose, cure, mitigate or prevent disease. SaMD is typically used with non-medical computing platforms connected to virtual networks or other general-use hardware.
2017
- (FDA & IMDRF-SaMD, 2017) ⇒J. Patrick Stewart, and Software as a Medical Device Working Group (2017). "Software as a Medical Device (SAMD): Clinical Evaluation – Guidance for Industry and Food and Drug Administration Staff". In: International Medical Device Regulators Forum (IMDRF).
- QUOTE: This document describes a converged approach for planning the process for clinical evaluation of a SaMD (software with a medical purpose as defined in SaMD N10), as illustrated in Figure 1, to establish that:
- There is a valid clinical association between the output of a SaMD and the targeted clinical condition (to include pathological process or state); and
- That the SaMD provides the expected technical and clinical data.
- QUOTE: This document describes a converged approach for planning the process for clinical evaluation of a SaMD (software with a medical purpose as defined in SaMD N10), as illustrated in Figure 1, to establish that:
| Clinical Evaluation | ||
| Valid Clinical | Association Analytical | Validation Clinical Validation |
| Is there a valid clinical association between your SaMD output and your SaMD’s targeted clinical condition? | Does your SaMD correctly process input data to generate accurate, reliable, and precise output data? | Does use of your SaMD’s accurate, reliable, and precise output data achieve your intended purpose in your target population in the context of clinical care? |
2015
- (IMDRF-SaMD, 2017) ⇒ Toshiyoshi Tominaga, and Software as a Medical Device Working Group (2015). "Software as a Medical Device (SaMD): Application of Quality Management System". In: International Medical Device Regulators Forum (IMDRF).
- QUOTE: The manufacturing of SaMD, which is a software-only product, is primarily based on the development lifecycle activities often supported by the use of automated software development tools (build automation, use of source code management tools, etc.). These automated activities may in some cases replace discrete or deliberate activities (e.g., transfer of design to production) typically found in the manufacturing of hardware products. However, the principles in a QMS that provide structure and support to the lifecycle proceses and activities are still applicable and important to control the quality of SaMD.
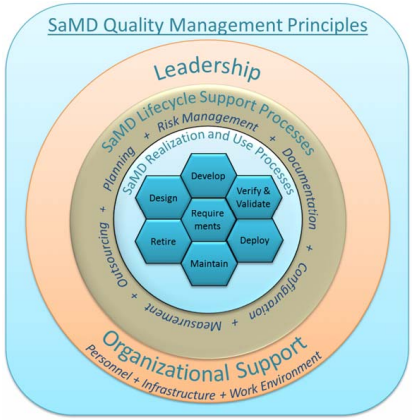
An effective QMS for SaMD should include the following principles:
- An organizational structure that provides leadership, accountability, and governance with adequate resources to assure the safety, effectiveness, and performance of SaMD (outer circle in Figure 1);
- A set of SaMD lifecycle support processes that are scalable for the size of the organization and are applied consistently across all realization and use processes (middle circle in Figure 1); and
- A set of realization and use processes that are scalable for the type of SaMD[1] and the size of the organization; and that takes into account important elements required for assuring the safety, effectiveness, and performance of SaMD (innermost circle in Figure 1).
- QUOTE: The manufacturing of SaMD, which is a software-only product, is primarily based on the development lifecycle activities often supported by the use of automated software development tools (build automation, use of source code management tools, etc.). These automated activities may in some cases replace discrete or deliberate activities (e.g., transfer of design to production) typically found in the manufacturing of hardware products. However, the principles in a QMS that provide structure and support to the lifecycle proceses and activities are still applicable and important to control the quality of SaMD.
|
- ↑ As identified by IMDRF SaMD WG N12 document
2014
- (IMDRF-SaMD, 2017) ⇒ Jeffrey Shuren, and Software as a Medical Device Working Group (2014). "Software as a Medical Device": Possible Framework for Risk Categorization and Corresponding Considerations". In: International Medical Device Regulators Forum (IMDRF).
- QUOTE: The following are necessary principles important in the categorization approach of SaMD.
- The categorization relies on an accurate and complete SaMD definition statement.
- The determination of the categories is the combination of the significance of the information provided by the SaMD to the healthcare decision and the healthcare situation or condition.
- The four categories (I, II, III, IV) are based on the levels of impact on the patient or public health where accurate information provided by the SaMD to treat or diagnose, drive or inform clinical management is vital to avoid death, long-term disability or other serious deterioration of health, mitigating public health.
- (...)
- QUOTE: The following are necessary principles important in the categorization approach of SaMD.
| State of Healthcare situation or condition | Significance of information provided by SaMD to healthcare decision | ||
|---|---|---|---|
| Treat or diagnose | Drive clinical management | Inform clinical management | |
| Critical | IV | III | II |
| Serious | III | II | I |
| Non-serious | II | I | I |
- Criteria for Category IV –
- i. SaMD that provides information to treat or diagnose a disease ]or conditions in a critical situation or condition is a Category IV and is considered to be of very high impact.
- Criteria for Category III –
- i. SaMD that provides information to treat or diagnose a disease or conditions in a serious situation or condition is a Category III and is considered to be of high impact.
- ii. SaMD that provides information to drive clinical management of a disease or conditions in a critical situation or condition is a Category III and is considered to be of high impact.
- Criteria for Category II –
- i. SaMD that provides information to treat or diagnose a disease or conditions in a nonserious situation or condition is a Category II and is considered to be of medium impact.
- ii. SaMD that provides information to drive clinical management of a disease or conditions in a serious situation or condition is a Category II and is considered to be of medium impact.
- iii. SaMD that provides information to inform clinical management for a disease or conditions in a critical situation or condition is a Category II and is considered to be of medium impact.
- Criteria for Category I –
- i. SaMD that provides information to drive clinical management of a disease or conditions in a non-serious situation or condition is a Category I and is considered to be of low impact.
- ii. SaMD that provides information to inform clinical management for a disease or conditions in a serious situation or condition is a Category I and is considered to be of low impact.
- iii. SaMD that provides information to inform clinical management for a disease or conditions in a non-serious situation or condition is a Category I and is considered to be of low impact.
- Criteria for Category IV –
2013
- (IMDRF-SaMD, 2013) ⇒ Despina Spanou, and Software as a Medical Device Working Group (2013). “Software as a Medical Device (SaMD): Key Definitions". In: International Medical Device Regulators Forum (IMDRF).
- QUOTE: The term “Software as a Medical Device” (SaMD) is defined as software intended to be used for one or more medical purposes that perform these purposes without being part of a hardware medical device.
- NOTES:
- SaMD is a medical device and includes in-vitro diagnostic (IVD) medical device.
- SaMD is capable of running on general purpose (non-medical purpose) computing platforms “without being part of” means software not necessary for a hardware medical device to achieve its intended medical purpose;
- Software does not meet the definition of SaMD if its intended purpose is to drive a hardware medical device.
- SaMD may be used in combination (e.g., as a module) with other products including medical devices;
- SaMD may be interfaced with other medical devices, including hardware medical devices and other SaMD software, as well as general purpose software.
- NOTES:
- QUOTE: The term “Software as a Medical Device” (SaMD) is defined as software intended to be used for one or more medical purposes that perform these purposes without being part of a hardware medical device.
- Mobile apps that meet the definition above are considered SaMD.